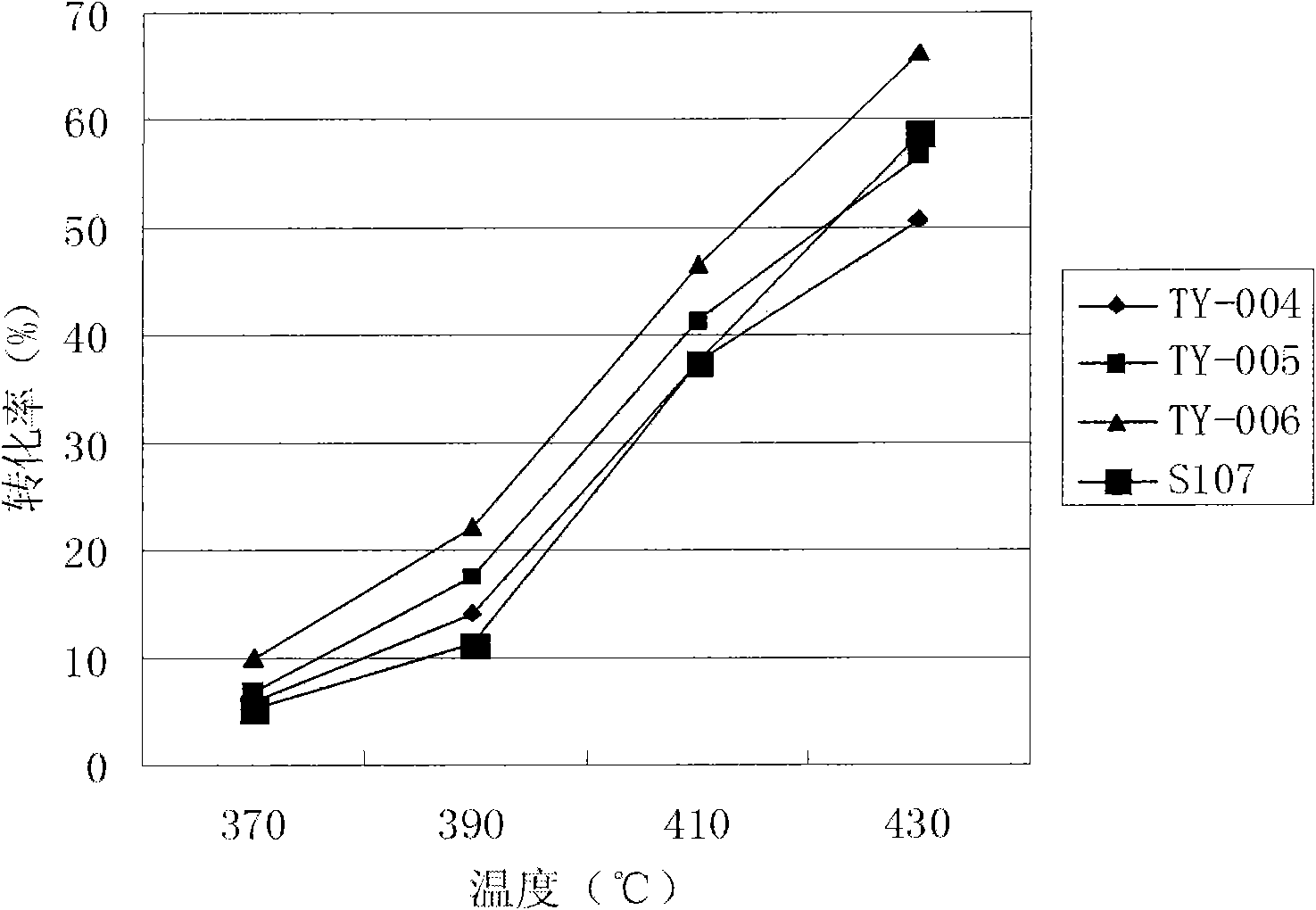
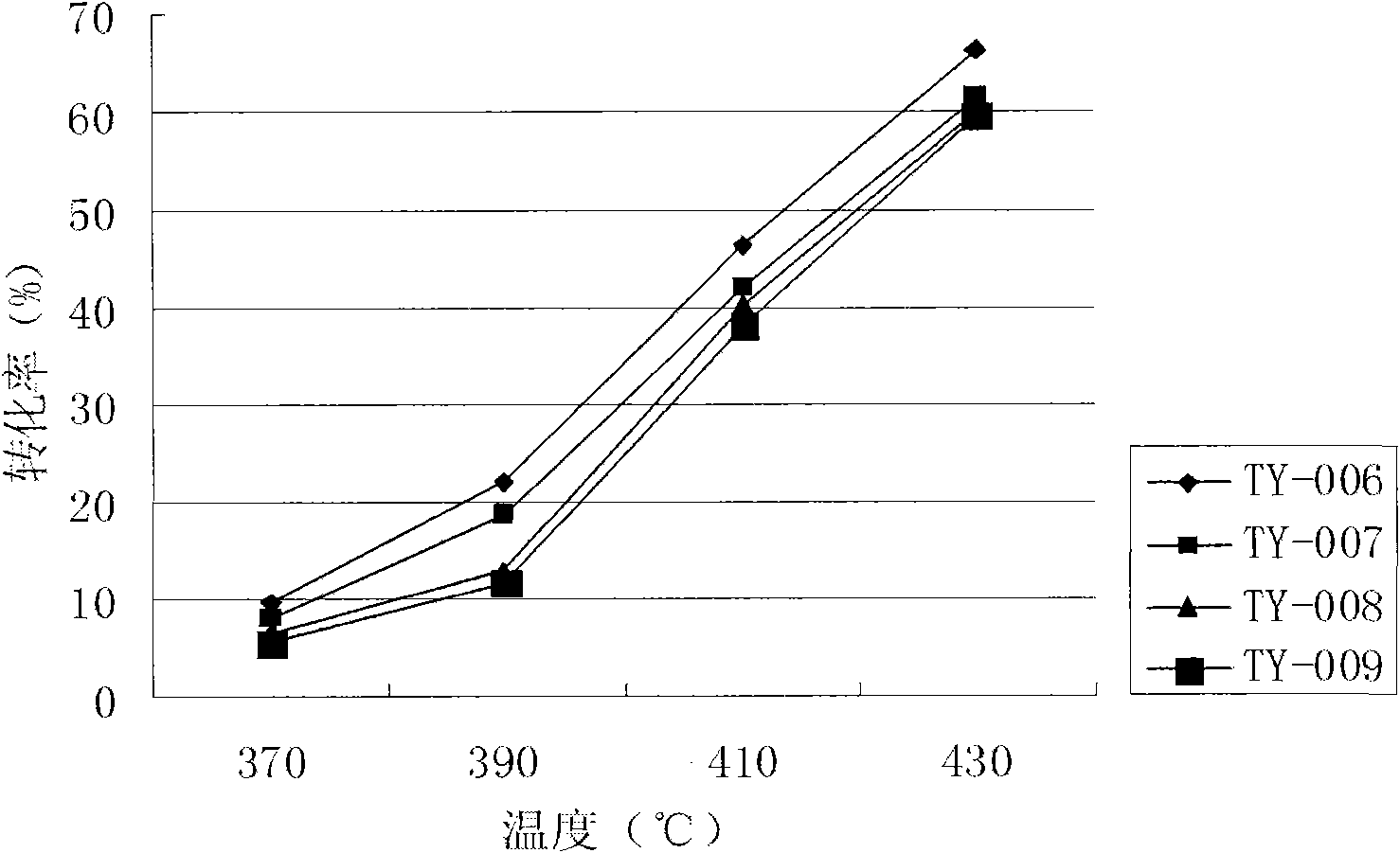
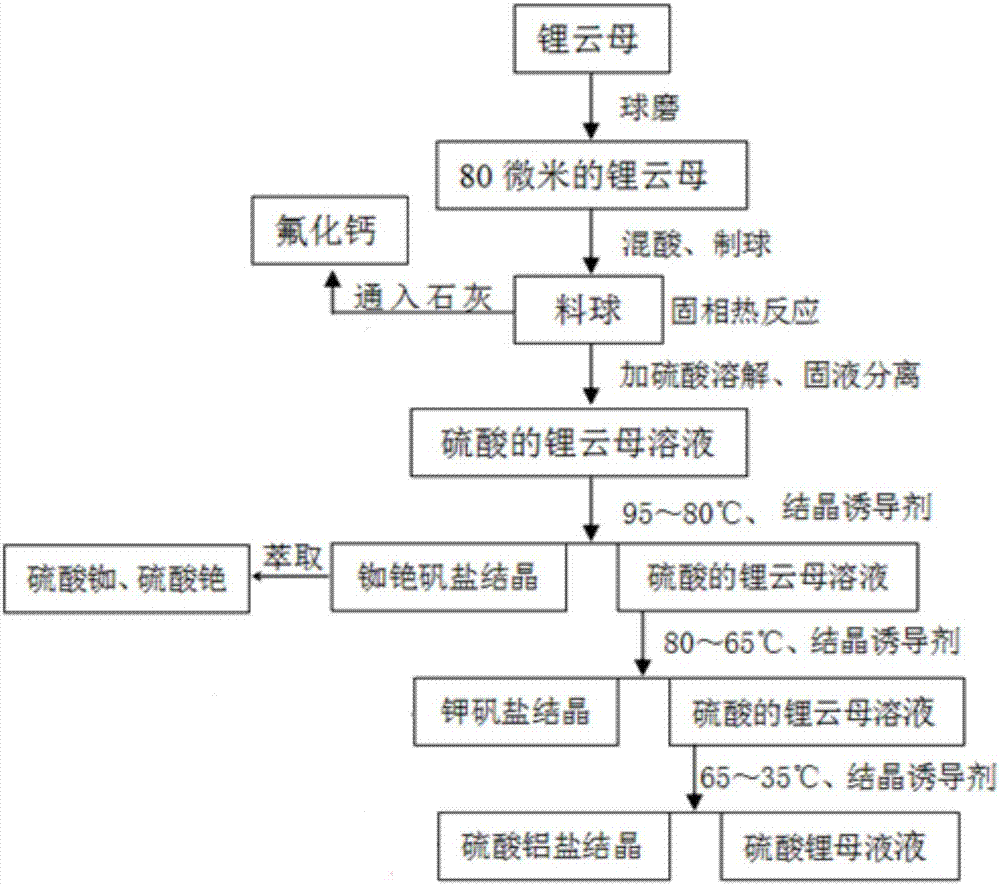
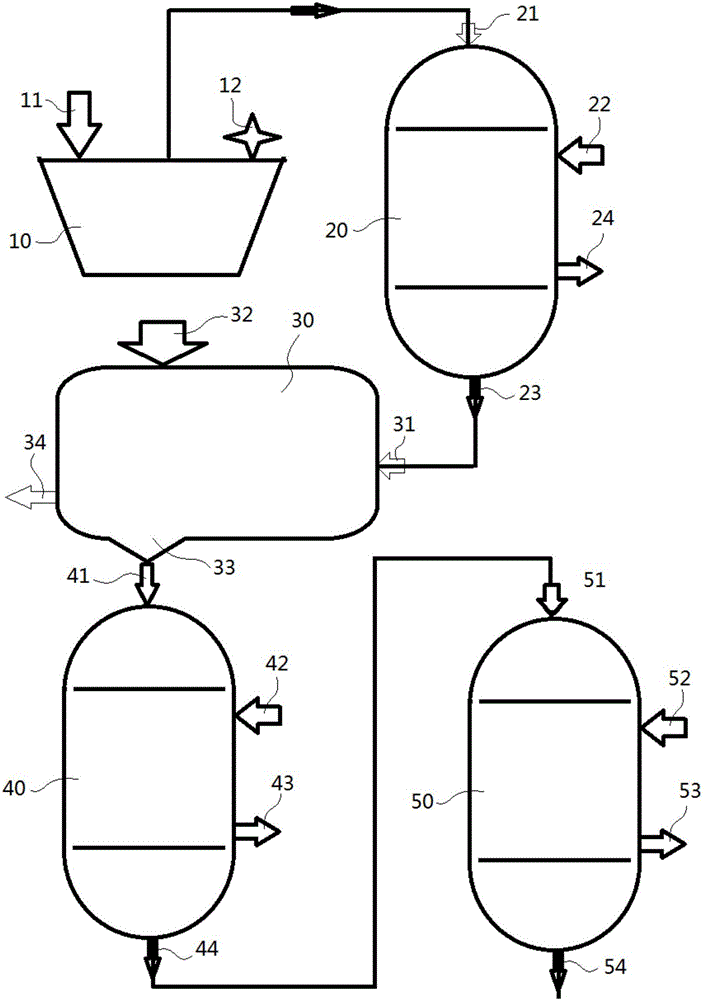
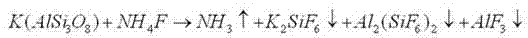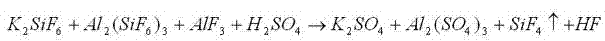Patents
Literature
42 results about "Cesium sulfate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Caesium sulfate or cesium sulfate is the inorganic compound and salt with the formula Cs 2 SO 4. It is a white water-soluble solid that is used to prepare dense aqueous solutions for use in isopycnic (or "density-gradient") centrifugation. It is isostructural with potassium salt.
Methods of making cesium salts and other alkali metal salts
A method of making a cesium salt is described and involves reacting a cesium sulfate containing solution with lime to form 1) a solution containing at least cesium hydroxide and 2) a residue comprising calcium sulfate. The method further involves removing the residue from the solution and converting the cesium hydroxide that is present in the solution to at least one type of cesium salt. The present invention further relates to uses of the cesium salt as well as methods of making cesium hydroxide using lime. Also, methods of making alkali metal salts and alkali metal hydroxides are also described.
Owner:CABOT SPECIALTY FLUIDS
Methods of making cesium salts and other alkali metal salts
A method of making a cesium salt is described and involves reacting a cesium sulfate containing solution with lime to form 1) a solution containing at least cesium hydroxide and 2) a residue comprising calcium sulfate. The method further involves removing the residue from the solution and converting the cesium hydroxide that is present in the solution to at least one type of cesium salt. The present invention further relates to uses of the cesium salt as well as methods of making cesium hydroxide using lime. Also, methods of making alkali metal salts and alkali metal hydroxides are also described.
Owner:CABOT SPECIALTY FLUIDS
Method for preparing aluminum cesium sulfate and aluminum rubidium sulfate by using tantalum-niobium tailings lepidolite
InactiveCN102173445AImprove utilization efficiencyReduce the impactCell electrodesAluminium sulfatesRubidium sulfateNiobium
The invention provides a method for preparing aluminum cesium sulfate and aluminum rubidium sulfate by using tantalum-niobium tailings lepidolite, which is characterized by comprising the steps of: smashing lepidolite powder into about 100 to 200 meshes; putting the lepidolite powder and a sulfuric acid solution with a concentration of 30 to 70 percent according to a solid-liquid mass ratio of 1:(2-8) into a reaction device; reacting for 3 to 10 hours at 60 to 200 DEG C to remove fluorine and obtain a sulfuric acid solution containing Li<+>, separating and removing a fluorine containing solution; filtering and separating the sulfuric acid solution containing Li<+>, washing filter residue with water fully to remove the filter residue and obtain filtrate which is mother solution 1; and changing the temperature of the mother solution 1 to 10 to 100 DEG C under stirring in order to separate solid aluminum cesium sulfate and solid aluminum rubidium sulfate.
Owner:宜春银锂新能源有限责任公司
Method for dissolving out to extract lithium from defluorinated lepidolite in pipeline
InactiveCN107739039AFast flowTake advantage ofRubidium/caesium/francium compoundsAlkali metal sulfites/sulfatesAlkaline earth metalSlurry
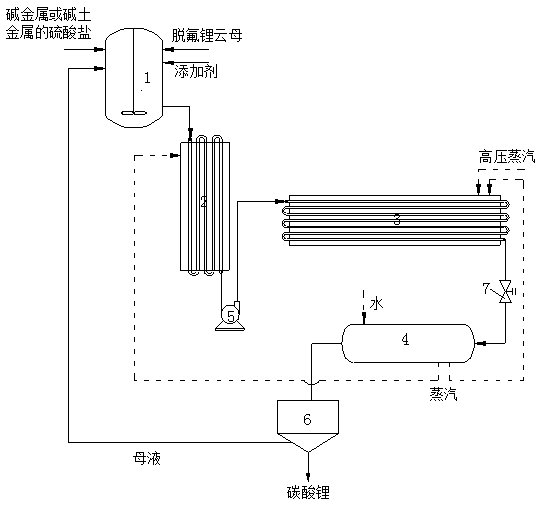
The invention relates to a method for dissolving out to extract lithium from defluorinated lepidolite in a pipeline. The method comprises the following steps: blending defluorinated lepidolite ore powder, sulfate of alkaline metal or alkaline earth metal, an additive and water according to the ratio to obtain slurry; after pre-heating the paste through a pre-heater, directly conveying the slurry into a pipeline reactor by utilizing a pump and carrying out high-temperature and high-pressure dissolving-out reaction; after reacting, cooling and recycling heat; carrying out solid-liquid separationon the slurry and washing; concentrating filtrate and removing impurities; adding sodium carbonate lithium-settling crystals to obtain a lithium salt product; circulating a lithium-settling mother solution until ingredients are blended to form slurry or sodium separation is carried out; then recycling potassium sulfate, rubidium sulfate and cesium sulfate. The method provided by the invention hasa simple system structure; compared with other lepidolite dissolving-out and lithium extraction technologies, the pipeline reactor is not provided with a mechanical stirring device and high pressureis easy to realize; the method has the advantages of less investment, low cost, small energy consumption and capability of comprehensively utilizing reaction dreg. The method is green and environmentally friendly, has high economic benefits and has an industrial production and application prospect.
Owner:FUZHOU UNIV
Method for producing potassium sulfate by utilizing insoluble rocks containing potassium
InactiveCN103539165AEfficient use ofReduce the temperatureSulfate/bisulfate preparationPotassium fertilisersNuclear chemistrySoluble solids
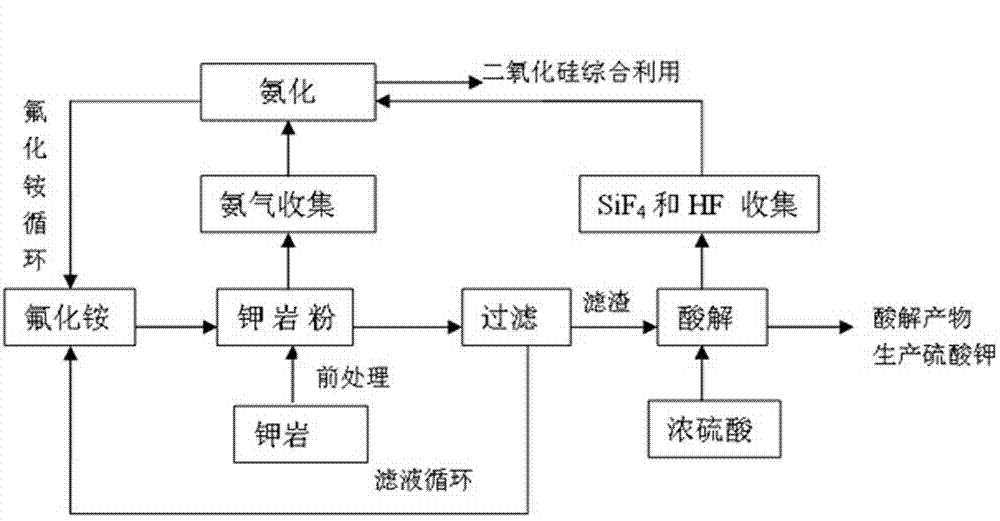
The invention relates to a method for producing potassium sulfate by utilizing insoluble rocks containing potassium. The technological process comprises the following steps: (1) pretreating the insoluble rocks containing potassium; (2) treating the insoluble rocks containing potassium by use of ammonium fluoride; (3) filtering a product treated with the ammonium fluoride; (4) performing acidolysis on the product obtained after treatment based on ammonium fluoride; (5) dissolving the product obtained after treatment based on sulfuric acid in water; (6) performing fractional precipitation by regulating the pH (Potential Of Hydrogen) so as to obtain a potassium sulfate solution; (7) regenerating and recycling an ammonium fluoride solution. Potash feldspars K(AlSi3O8) are mainly taken as the rocks containing potassium. Rubidium feldspars and cesium feldspars, which are utilized to replace the potash feldspars, are used for producing rubidium sulfate and cesium sulfate by utilizing the process and the process condition which are provided by the invention. The insoluble rocks containing potassium are converted into acid-soluble solids by use of ammonium fluoride, so that the potassium sulfate is obtained after the acid-soluble solids are acidified by the sulfuric acid; meanwhile, other elements in the rocks are effectively utilized. The ammonium fluoride is recycled in the whole technological process. The technological process is low in temperature, easy to control and high in reaction yield.
Owner:GUIZHOU YUANSHENG POTASSIUM TECH
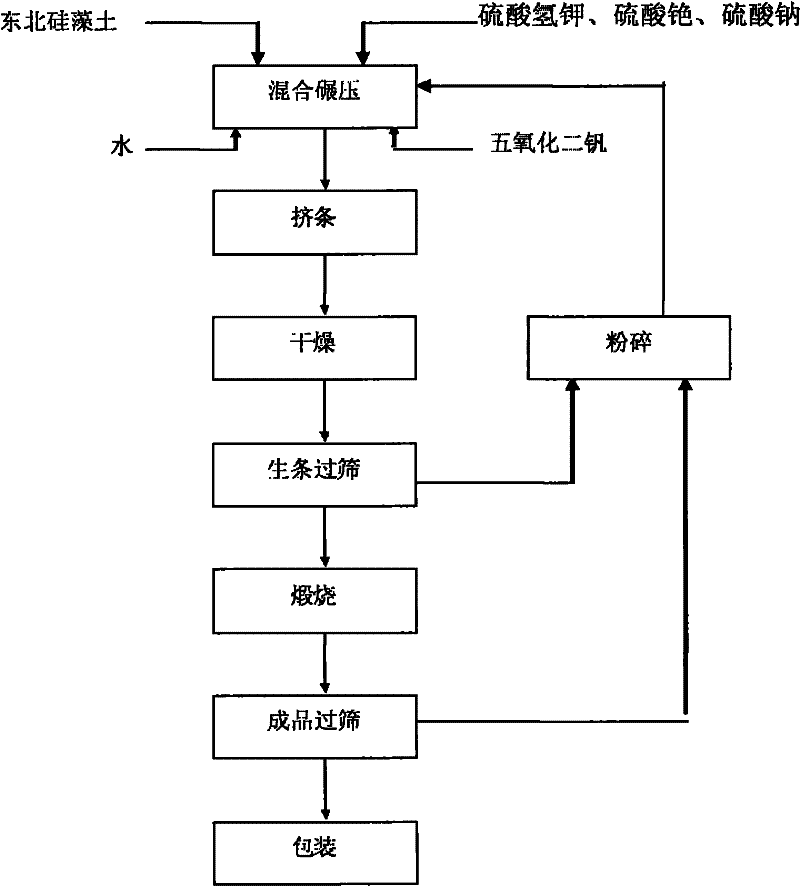
Technological process of preparing high efficient vanadium catalyst with nanometer vanadium material
InactiveCN101088607AHigh low temperature activitySimple preparation processSulfur-trioxide/sulfuric-acidMetal/metal-oxides/metal-hydroxide catalystsMicroparticleHigh activity
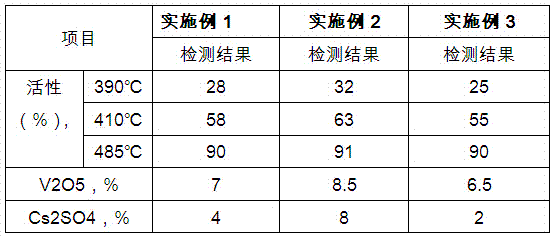
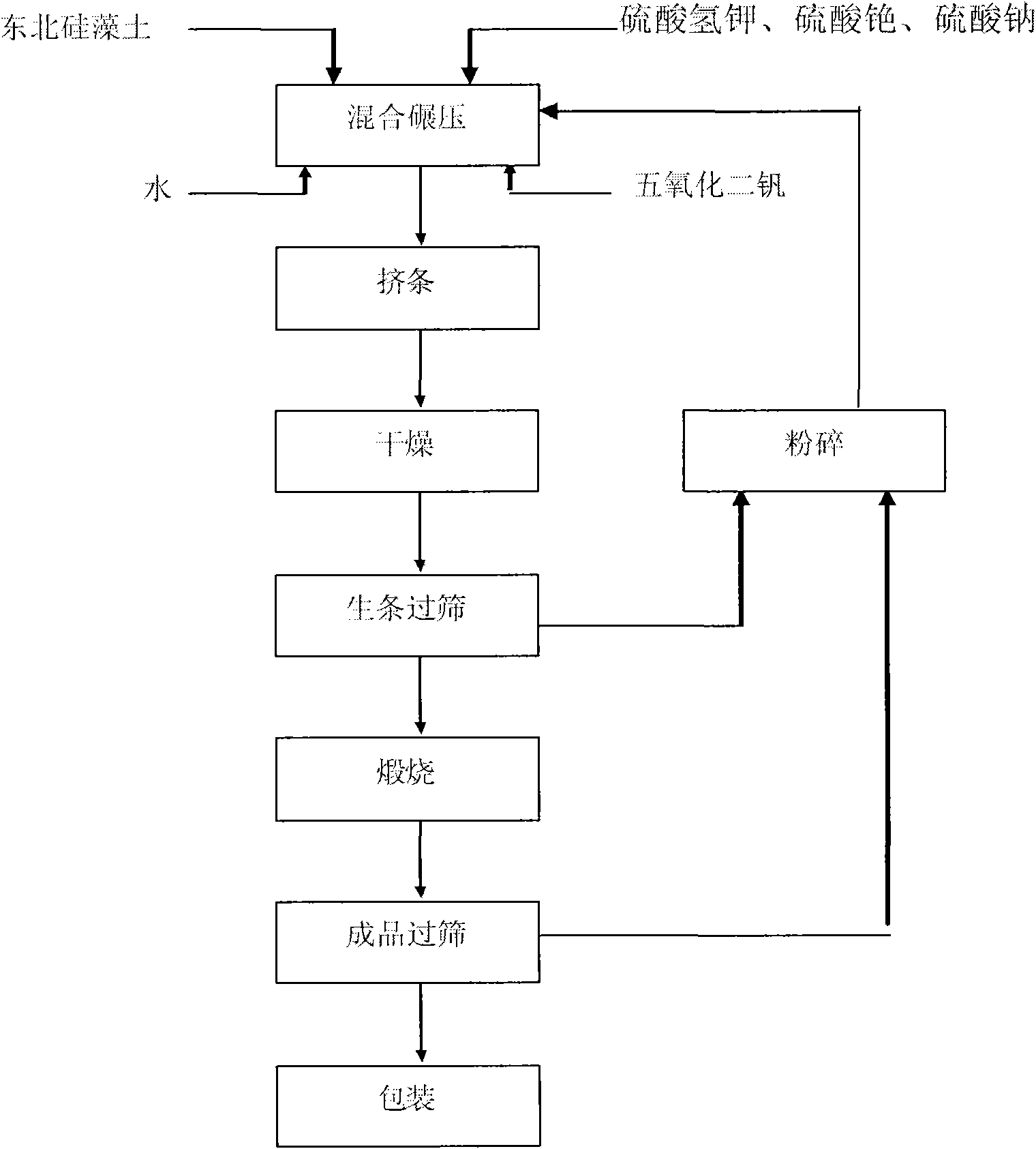
The present invention prepared nanometer vanadium catalyst with flaky industrial vanadium pentoxide crystal or pure vanadium pentoxide material, and through an inorganic sol-gel process to prepare colloid containing nanometer vanadium pentoxide particle, the subsequent mixing with carrier diatomite and co-catalyst potassium sulfate and cesium sulfate in a wet grinder, rolling, extruding to form, setting at room temperature for some time, drying in a drying box and final roasting in a muffle to obtain the catalyst. The catalyst is applied in sulfuric acid production, and has the features of low operation temperature, low power consumption and high activity.
Owner:PANZHIHUA UNIV
Catalyst containing caesium and vanadium and preparation method thereof
InactiveCN105435772AExtended service lifeQuality improvementSulfur compoundsMetal/metal-oxides/metal-hydroxide catalystsSulfite saltPotassium hydroxide
The invention discloses a catalyst containing caesium and vanadium and a preparation method thereof. With the total mass of the catalyst components as 100% parts, the content of vanadium pentoxide is 5-10%, the content of cesium oxide is 2-14%, the content of potassium oxide is 6-14%, the content of sodium oxide is 1-9%, and the balance is diatomaceous earth. A raw material of the cesium oxide is salt or alkali containing the cesium, such as cesium sulfate (Cs2SO4), cesium carbonate (Cs2CO3) or cesium hydroxide (CsOH). A raw material of the potassium oxide is salt or alkali containing potassium like potassium sulfate, potassium sulfite or potassium hydroxide. A raw material of the sodium oxide is salt or alkali containing sodium like sodium sulfate, sodium sulfite or sodium hydroxide. The catalyst is stable in performance, low in operating temperature, wide in areas, slow in heat fading and high in catalytic efficiency.
Owner:贵州威顿催化技术有限公司
Method for recovering rubidium, caesium, aluminum and potassium from mixed alum by-produced during extraction of lithium from lithionite
ActiveCN108996532APrevent dehydrationReduce manufacturing costPotassium sulfate preparationRubidium/caesium/francium compoundsRubidium sulfateCesium sulfate
The invention discloses a method for recovering rubidium, cesium, aluminum and potassium from mixed alum by-produced during extraction of lithium from lithionite. According to the invention, the byproduct, i.e., the mixed alum, obtained after the extraction of lithium from lithionite is used as a raw material, and a plurality of products like rubidium sulfate and cesium sulfate are separately extracted. The method comprises the following steps: 1) a hybrid reaction; 2) preparation of aluminum sulfate crystals; and 3) refining of rubidium sulfate and cesium sulfate crystals. The operation of the method is safe and easily practicable. Compared with traditional methods normally using organic solvent extraction for production of rubidium and cesium salts, the method provided by the invention has the following advantages: the comprehensive utilization rate of lithionite is increased, the production cost of lithium salt extraction is lowered, harm to environment during lithium extraction isreduced; and equipment investment is lowered.
Owner:江西海汇龙洲锂业有限公司
Low-temperature type vanadium catalyst for catalyzing SO2 oxidizing reaction
ActiveCN101850261AGood catalytic activity at low temperatureMeet industry needsPhysical/chemical process catalystsSulfur compoundsSulfateCesium sulfate
The invention provides a low-temperature type vanadium catalyst for catalyzing SO2 oxidizing reaction, which comprises kieselguhr, vanadium pentoxide, potassic sulfate, sodium-contained sulfate and cesium sulfate. The catalyst has excellent catalytic activity. The invention also provides a preparation method of the vanadium catalyst for catalyzing the SO2 oxidizing reaction. The method directly adopts a mixed milling method to prepare the vanadium catalyst without acid treatment or a vanadium oxidizing process or a neutralization process, and thereby, not only is the production flow shortened but also acidic waste water can not be generated. The catalyst has environmental friendliness.
Owner:吉林省临江天元催化剂有限公司
Method for extracting aluminum sulfate, aluminum potassium sulfate, rubidium sulfate and cesium sulfate by aid of leaching agents of lithium mica sulfuric acid
ActiveCN107140667AAvoid disadvantages such as not easy to useRubidium/caesium/francium compoundsAluminium sulfatesBall millSolid phases
The invention provides a method for extracting aluminum sulfate, aluminum potassium sulfate, rubidium sulfate and cesium sulfate by aid of leaching agents of lithium mica sulfuric acid. The method includes the steps of mica ball mill activation, solid phase thermal reaction of sulfuric acid, thermal extraction of dilute sulphuric acid, continuous crystallization and preparation of rubidium sulfate and cesium sulfate to obtain aluminum sulfate, aluminum potassium sulfate and cesium sulfate in sequence. The aluminum sulfate, aluminum potassium sulfate and cesium sulfate can be obtained from the preparation method, and the aluminum sulfate and the aluminum potassium sulfate which are extracted can be sold directly in industrial-grade products after disacidifying, washing and drying; in addition, the preparation method is simple in steps, and shortcomings of more waste, difficulty in use of by-product and the like can be overcome.
Owner:江西南氏锂电新材料有限公司
Method and system for extracting rubidium salt and cesium salt from mother liquor after extracting lithium from lepidolite
InactiveCN106379922AHigh recovery rateHigh extraction control conditionsRubidium/caesium/francium compoundsSeparation coefficientRubidium
The invention provides a method for extracting rubidium salt and cesium salt from mother liquor after extracting lithium from lepidolite, which is characterized by comprising the following steps: 1) adjusting a high salinity solution into an alkaline solution; 2) extracting rubidium ions and caesium ions in the alkaline solution obtained in the step 1) by adopting an organic extractant, so as to obtain a loaded organic phase I and raffinate; 3) washing the organic phase I obtained in the step 2), so as to obtain an organic phase II and washing liquor; 4) carrying out back extraction on the loaded organic phase II with back extraction acid I, so as to obtain rubidium salt back extraction liquor and a cesium ion-loaded organic phase; and 5) carrying out back extraction on the cesium ion-loaded organic phase obtained in the step 4) with back extraction acid II, so as to obtain cesium salt back extraction liquor and a blank organic phase. According to the invention, the mother liquor after extracting lithium from lepidolite is controlled to be a strong alkali state, and the solution after back extraction has relatively low impurity content, so that the separation coefficient is high. According to the invention, the mother liquor after extracting lithium from lepidolite is controlled to be a strong alkali state, and the extraction becomes complete, so that the recovery rate is high.
Owner:JIANGXI RARE EARTH & RARE METALS TUNGSTEN GRP HLDG CO LTD
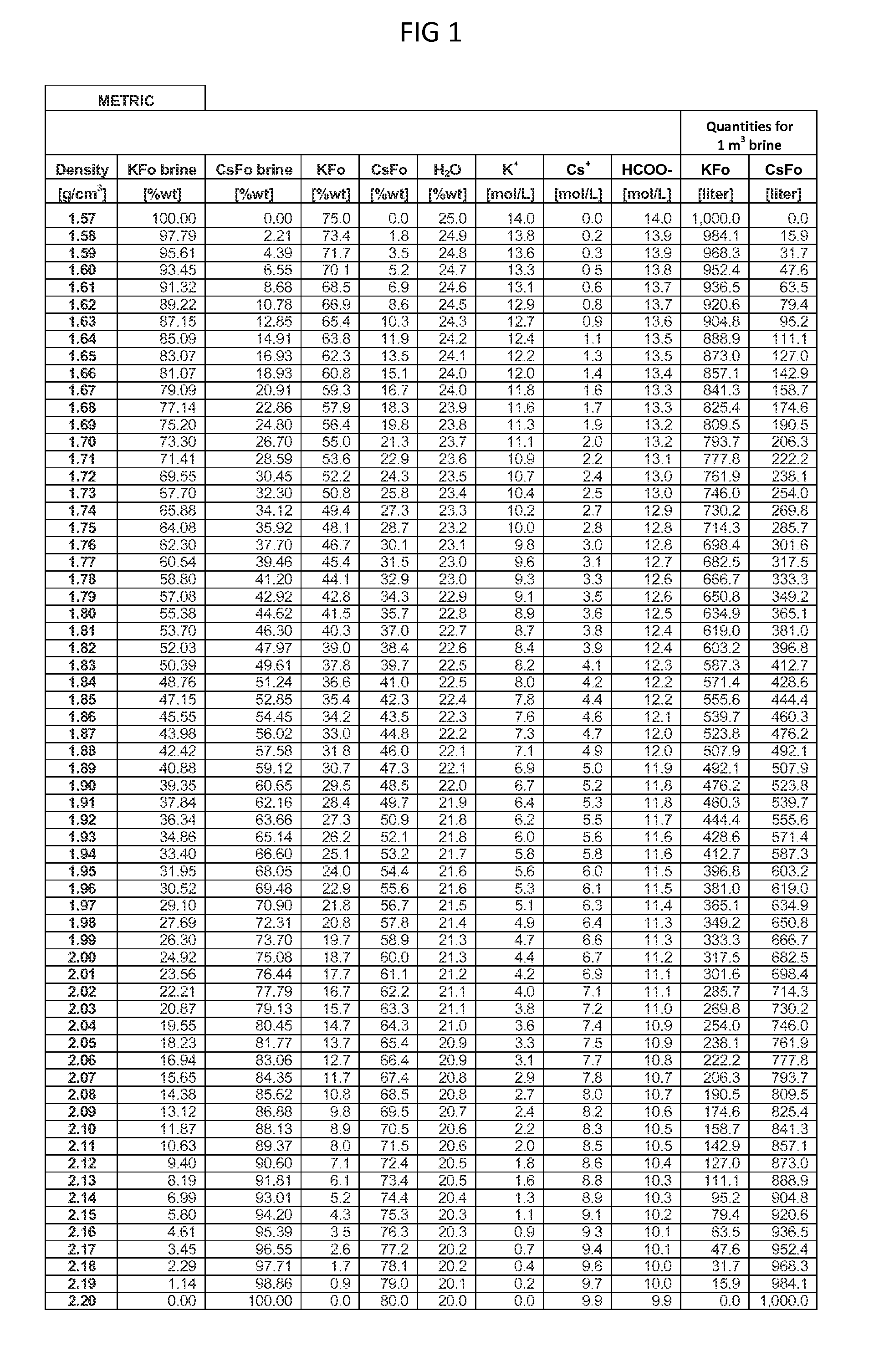
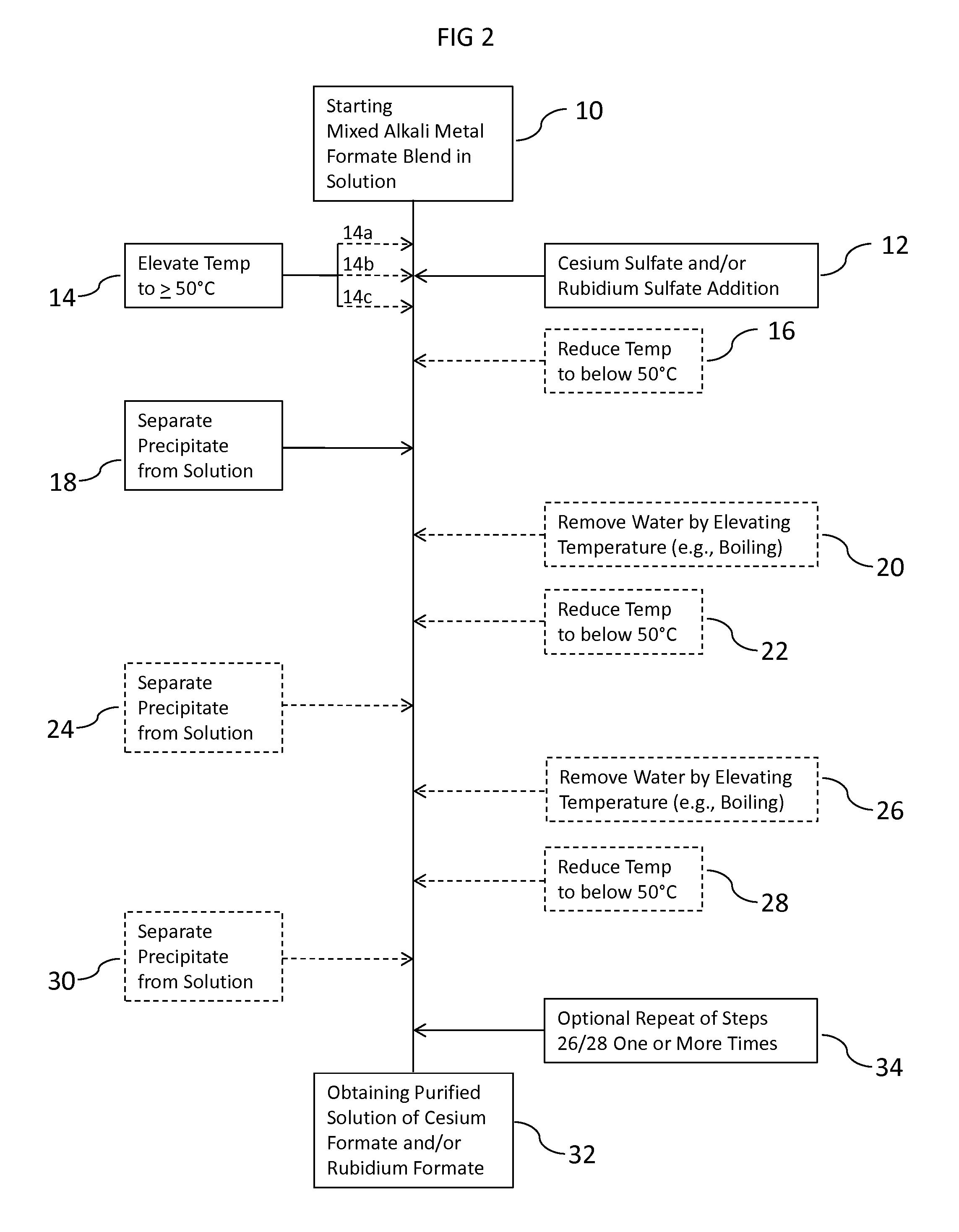
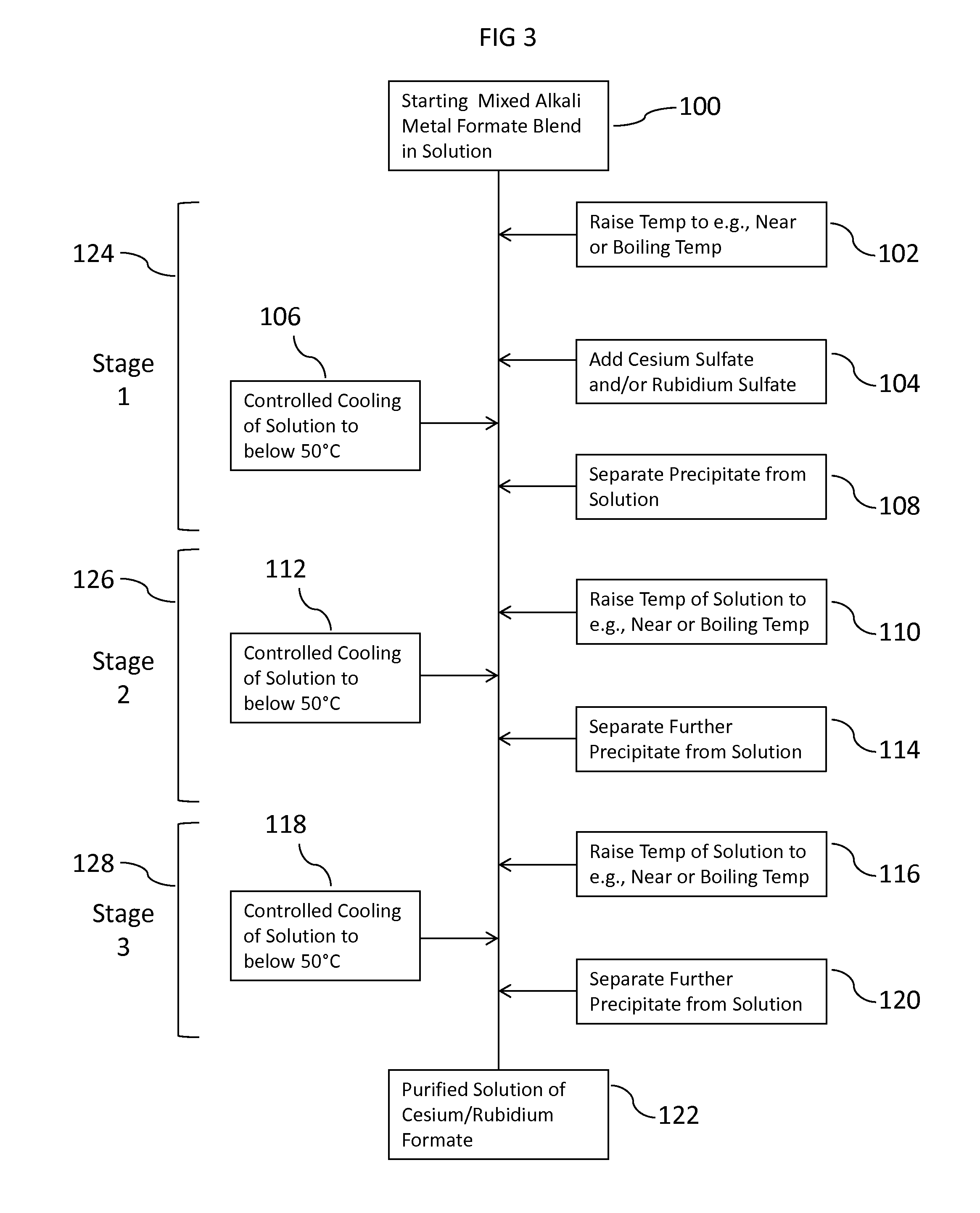
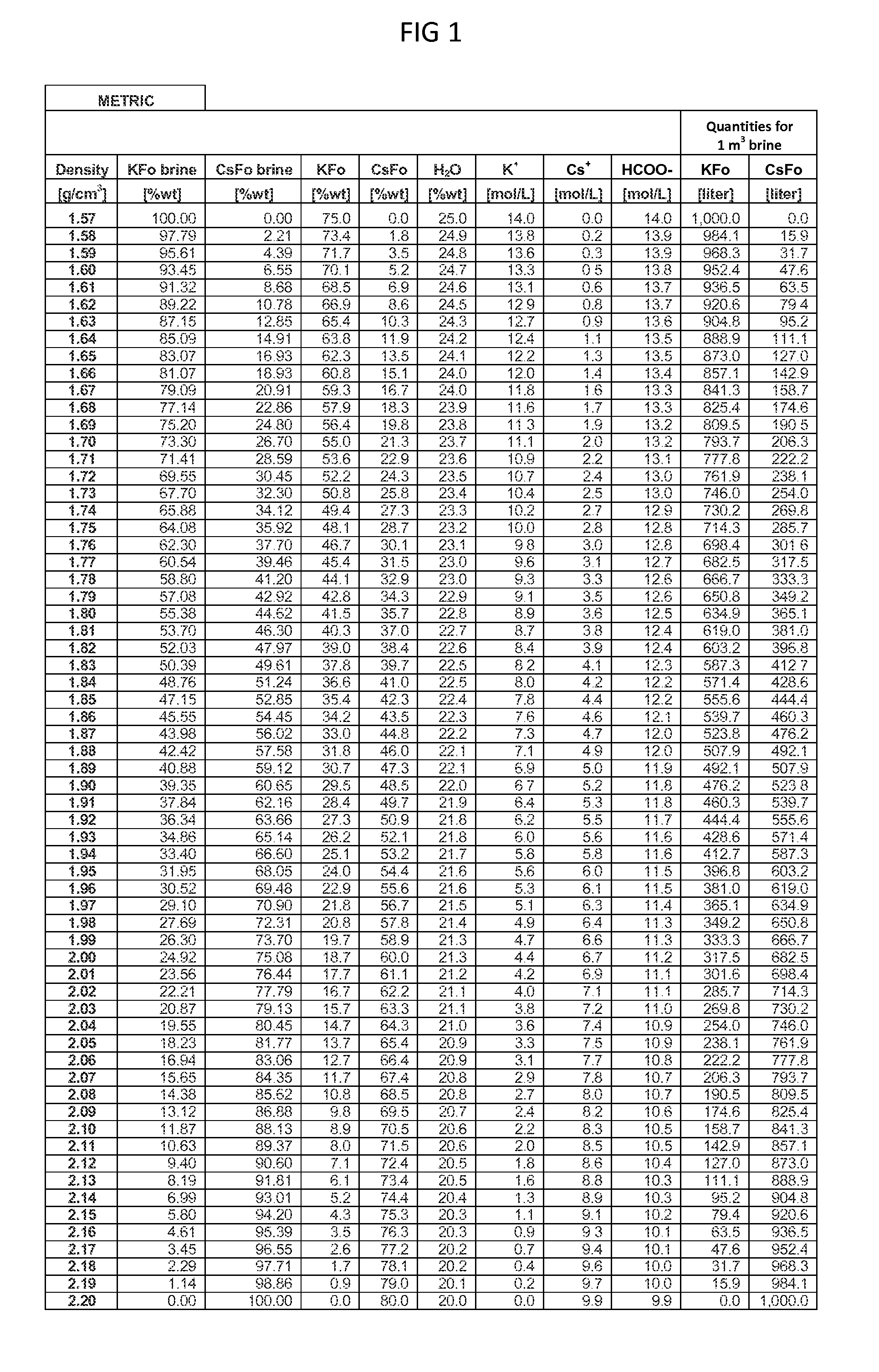
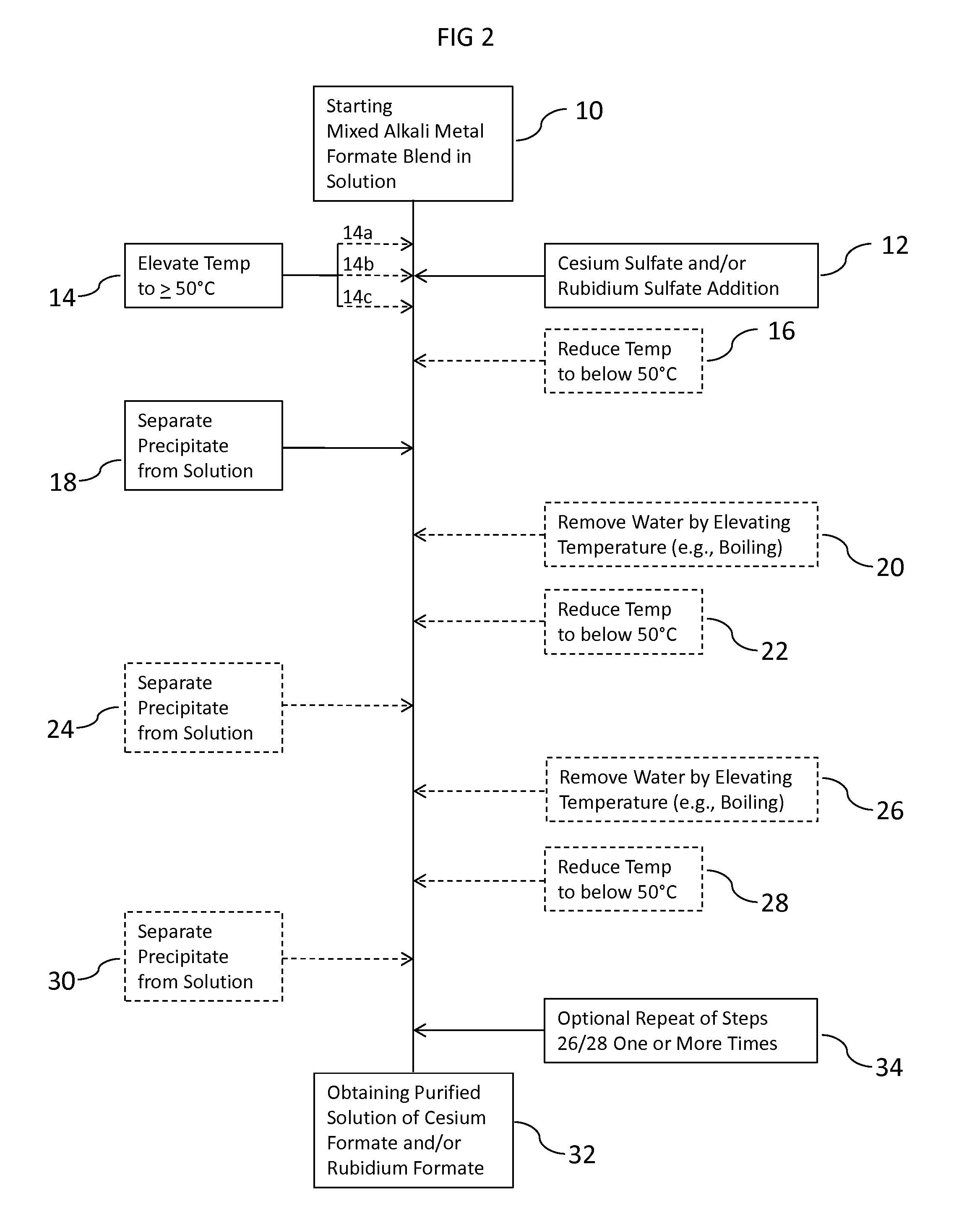
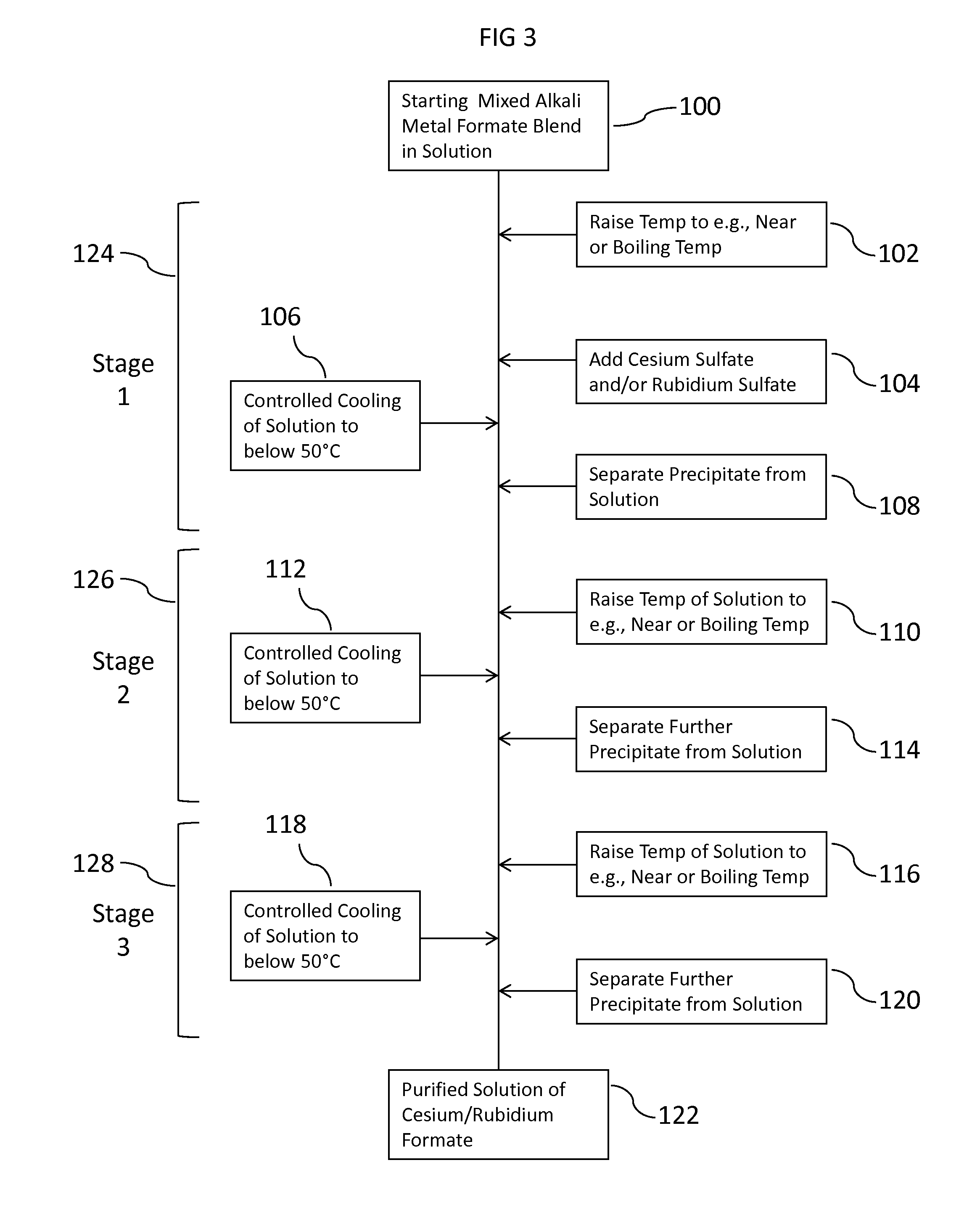
Methods To Recover Cesium Formate From A Mixed Alkali Metal Formate Blend
ActiveUS20150152033A1Efficient and inexpensivePreparation from carboxylic acid saltsOrganic compound preparationSodium bicarbonateFormate
Methods to recover or separate cesium formate or rubidium formate or both from a mixed alkali metal formate blend are described. One method involves adding cesium sulfate or rubidium sulfate to the mixed alkali metal formate blend in order to preferentially precipitate potassium sulfate from the mixed alkali metal formate blend. Another method involves adding cesium carbonate or cesium bicarbonate or both to preferentially precipitate potassium carbonate / bicarbonate and / or other non-cesium or non-rubidium metals from the mixed alkali metal blend. Further optional steps are also described. Still one other method involves converting cesium sulfate to cesium hydroxide.
Owner:CABOT SPECIALTY FLUIDS
Method for preparing cesium carbonate by ion exchange method
ActiveCN103241750AWide range of market sourcesRaw materials are easy to getRubidium/caesium/francium compoundsCesium sulfateIon exchange
The invention relates to the technical field of a preparation method of cesium carbonate and in particular relates to a method for preparing cesium carbonate by an ion exchange method. The method is applicable to preparation of high-purity pollution-free cesium carbonate. The method comprises the following steps of: performing acid leaching, jarosite precipitation, recrystallization and conversion on pollucite to obtain a conversion solution of cesium sulfate; exchanging by using 201*7 strongly alkaline styrene anion exchange resin to obtain a cesium hydroxide solution; and introducing carbon dioxide into the cesium hydroxide solution and neutralizing to prepare a caesium bicarbonate solution; and concentrating, crystallizing, separating and drying to obtain the cesium carbonate finished product. The obtained product is high in purity, simple in process flow, economic and environment-friendly, and suitable for industrialized production; and the process adopting the anion exchange resin is simpler than the process adopting cation exchange resin and elution is avoided, so cesium loss in an eluting process is avoided, the system yield is increased, and the system yield after a mother liquid participates in circulation can reach 92 to 95 percent.
Owner:百杰瑞(荆门)新材料有限公司
Preparation method of cesium sulfate and rubidium sulfate
InactiveCN110550646AEasy to handleEasy to recycleRubidium/caesium/francium compoundsRubidium sulfateLithium
The invention relates to the technical field of mineral extraction, in particular to a preparation method of cesium sulfate and rubidium sulfate. A traditional cesium sulfate extraction method is improved, cesium is extracted from lithium-precipitation waste residue firstly by taking t-BAMBP and 260# solvent oil with the specific ratio as extraction solutions and through a multistage extraction technology, a cesium sulfate pure product is obtained, and the product purity reaches up to 99.9% or above; and then rubidium and potassium can be separated from raffinate of cesium extraction by adopting the t-BAMBP and n-hexane with the specific ratio as extraction solutions, the high-purity rubidium sulfate is obtained, and the purity can reach up to 99.9% or above. The preparation process involves a few types of organic solvents, and waste liquid is treated, recycled and reused advantageously.
Owner:YICHUN KEYUAN CHEM CO LTD
Methods of making cesium salts and other alkali metal salts
A method of making a cesium salt is described and involves reacting a cesium sulfate containing solution with lime to form 1) a solution containing at least cesium hydroxide and 2) a residue comprising calcium sulfate. The method further involves removing the residue from the solution and converting the cesium hydroxide that is present in the solution to at least one type of cesium salt. The present invention further relates to uses of the cesium salt as well as methods of making cesium hydroxide using lime. Also, methods of making alkali metal salts and alkali metal hydroxides are also described.
Owner:SINOMINE RESOURCE EXPLORATION
Formula and preparation method of ferrite permanent magnetic material
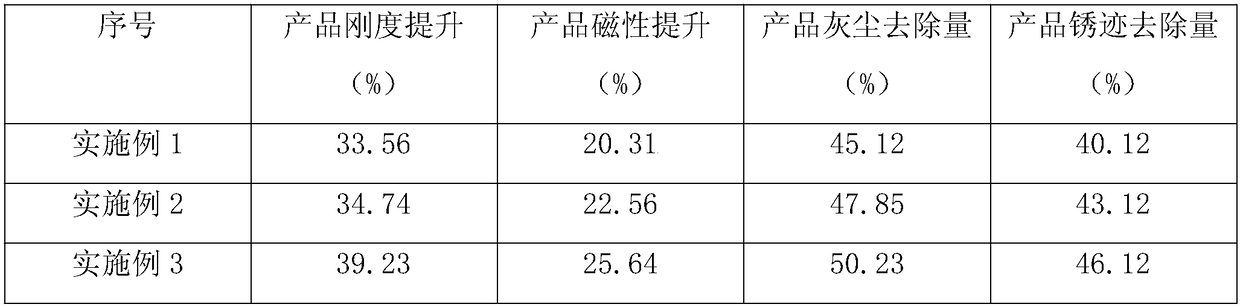
InactiveCN108083793AIncreased degree of anisotropic orientationGood removal effectInorganic material magnetismInductances/transformers/magnets manufactureCesium sulfateGrain growth
The invention discloses a formula and preparation method of a ferrite permanent magnetic material. The ferrite permanent magnetic material comprises, by weight, 60 to 80% of iron oxide red, 10 to 15%of barium carbonate, 5 to 10% of silica, 3 to 8% of calcium carbonate, 5 to 10% of cesium sulfate, 15 to 20% of titanium dioxide and 5 to 10% of a rust remover. Through use of calcium carbonate, the solid-phase reaction is promoted, the product sintering temperature is reduced and the density of the product is improved. Through use of cesium sulfate, the anisotropic orientation of the ferrite is obviously improved, the crystal grain growth is inhibited and coercivity is improved. Through use of the titanium dioxide and rust remover, removal of rust on the surface of the ferrite permanent magnetic material is promoted and the influence of rust on magnetic properties is prevented.
Owner:宁波市鄞州智伴信息科技有限公司
Method for preparing cesium nitrate by pollucite
The invention belongs to the technical field of cesium nitrate preparations and particularly discloses a method for preparing cesium nitrate by pollucite. The method includes that the pollucite is subjected to acid leaching, jarosite precipitation, recrystallization and conversion to obtain a conversion solution of cesium sulfate, a dilute solution of the cesium nitrate is obtained after a double decomposition reaction of calcium nitrate and an impurity removing by adding barium hydroxide and carbon dioxide, and ultimately a finished product of the cesium nitrate is prepared by an evaporation crystallization and a drying. The direct recovery rate of the obtained cesium nitrate product is about 89%, the cesium nitrate content in a product mother liquor is approximately 10.27%, the cesium content in calcium sulfate of a conversion slag after being washed is about 0.48%, the cesium content in a barium sulfate slag is about 0.25%, and the total recovery rate of the mother liquor participating in the recycling is approximately 95.5%.
Owner:HUBEI BAIJIERUI ADVANCED MATERIALS
Difluoro antimony cesium sulfate nonlinear optical crystal and preparation method and application thereof
ActiveCN109295494AShort UV cut-off edgeUV absorption cut-off edge is largePolycrystalline material growthFrom normal temperature solutionsNonlinear optical crystalFiber
The invention discloses a difluoro antimony cesium sulfate nonlinear optical crystal and a preparation method and application thereof. A room-temperature solution method is adopted for preparing the difluoro antimony cesium sulfate nonlinear optical crystal, wherein the molecular formula of the crystal belonging to an orthorhombic system is CsSbSO4F2, the molecular weight is 388.72, and the spacegroup is Pna21; the cell parameters include: a=9.9051(5) , b=11.6070(8) , c=5.2822(3) , and V=607.29(6) <3>; the nonlinear optical effect of the crystal is about 0.65KDP, the ultraviolet cut-off side is 233nm, and high rate in penetration of visible light and near infrared band is achieved. The CsSbSO4F2 crystal is transparent and free of inclusion and has advantages of high growth speed, lowcost, stable physicochemical properties, high mechanical performances, easiness in processing and the like, and the crystal is suitable for manufacturing of nonlinear optical devices such as frequencymultipliers, secondary harmonic generators, up-down frequency converters, optical parameter oscillators, laser blinding weapons, laser discs, laser projection televisions and optical computation andoptical fiber communication devices.
Owner:MINJIANG UNIV
Barrier film material and pattern formation method using the same
InactiveUS20060127812A1Good body shapePrevent elutionRadiation applicationsSemiconductor/solid-state device manufacturingResistCesium sulfate
A resist film is first formed on a substrate. Subsequently, a barrier film including a basic compound of, for example, dicyclohexylamine is formed on the resist film. Thereafter, with an immersion liquid including cesium sulfate provided on the barrier film, pattern exposure is carried out by selectively irradiating the resist film with exposing light through the barrier film. Then, after removing the barrier film, the resist film having been subjected to the pattern exposure is developed, so as to form a resist pattern in a good shape.
Owner:RPX CORP
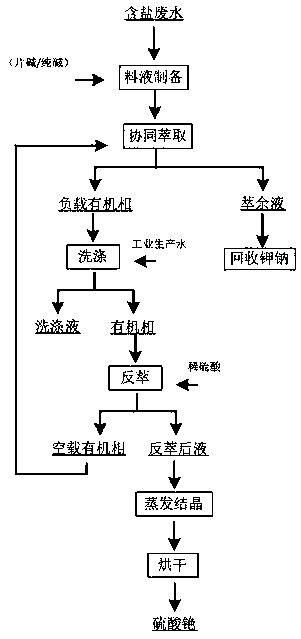
Method for preparation cesium sulfate from salt-containing wastewater
ActiveCN109536740AQuality assuranceEfficient separationProcess efficiency improvementCesium sulfateKerosene
The invention relates to the field of recovering high-value products from industrial waste, in particular to a method for preparing cesium sulfate from salt-containing wastewater. The method comprisesthe following steps that firstly, an extraction agent is prepared, t-BAMBP, P204 and kerosene are taken, and the t-BAMBP, the P204 and the kerosene are mixed to prepare the extraction agent; then theconcentration of material liquid ions is detected, then the salt-containing wastewater is taken for adjusting the PH value, and then multistage countercurrent extraction is carried out on the material liquid by using the extraction agent so as to prepare a load organic phase; then circulation countercurrent washing is carried out on the load organic phase by using a water washing solution, then aback-extraction agent is prepared, and then back extraction is carried out; and finally evaporation is carried out to prepare the cesium sulfate. Thus, the effect that cesium elements are separated and extracted from the salt-containing wastewater is achieved, and a method for extracting the cesium in the salt-containing wastewater which is based on the synergism of the t-BAMBP and the P204 underan alkaline condition is provided by utilizing the alkalescence of the salt-containing wastewater.
Owner:GREENNOVO ENVIRONMENTAL TECH CO LTD
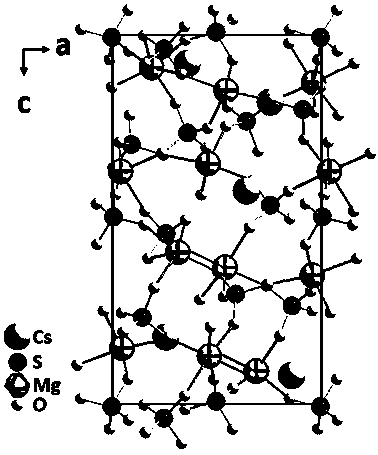
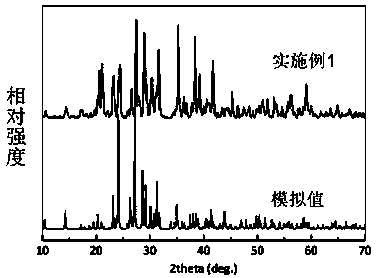
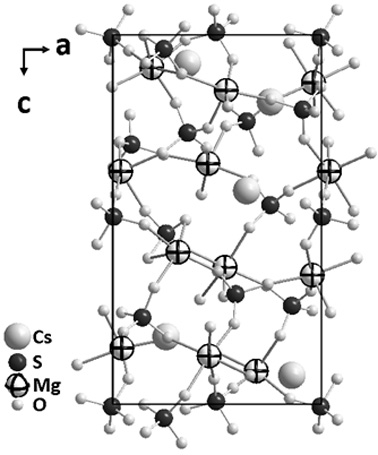
Cesium sulfate magnesium non-linear optical crystal as well as preparation method and application thereof
ActiveCN110079858APhysicochemically stableModerate hardnessPolycrystalline material growthFrom frozen solutionsNonlinear optical crystalSpace group
The invention discloses a Cs2Mg3(SO4)4 non-linear optical crystal as well as a preparation method and application thereof. The preparation method comprises the following step: by taking Cs2SO4 and Mg-containing sulfate as raw materials, sintering so as to obtain a Cs2Mg3(SO4)4 microcrystal; or mixing and melting Cs2SO4 and Mg-containing sulfate, thereby obtaining the Cs2Mg3(SO4)4 non-linear optical crystal. The Cs2Mg3(SO4)4 disclosed by the invention belongs to an orthorhombic system P212121 space group, a MgO6 polyhedron and a SO4 tetrahedron in the structure are in co-top point connection toform a three-dimensional frame, and in addition, frame pores are filled with Cs<+> ions. By adopting the crystal, a pure phase and a single crystal of Cs2Mg3(SO4)4 are obtained, the synthesis methodis simple, the yield is high, and the crystal has a short ultraviolet cut-off side, a moderate frequency doubling conversion efficiency and good growth habits, and can be applied to non-linear opticaldevices such as frequency conversion, optical parameter oscillation, electro-optical modulation and communication.
Owner:MINJIANG UNIV
Preparation method of high-dispersion nano cluster vanadium catalyst
ActiveCN112808262AHigh compressive strengthImprove distributionCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsOXALIC ACID DIHYDRATEPtru catalyst
The invention discloses a preparation method of a high-dispersion nano cluster vanadium catalyst, which comprises the following steps: dissolving vanadium pentoxide solid in 98% concentrated sulfuric acid, and heating and stirring to fully dissolve the vanadium pentoxide solid; adding oxalic acid solid, and fully reacting to convert the oxalic acid solid into a vanadyl sulfate solution; adding nano scale silicon dioxide powder and distilled water, and stirring at a constant temperature, so that the nano scale silicon dioxide powder and the distilled water are fully mixed with the vanadyl sulfate solution; adding sodium sulfate, potassium sulfate, potassium pyrosulfate and cesium sulfate, fully dissolving the alkali metal salt to obtain catalyst slurry, pouring the catalyst slurry into diatomite, fully mixing and dispersing the catalyst slurry in the diatomite, molding, and drying until the moisture is less than 10% to obtain a catalyst semi-finished product; and roasting in a muffle furnace at the temperature of 550-650 DEG C for 45 minutes to obtain the vanadium catalyst. The catalyst has high activity and high thermal stability, and can effectively reduce the emission of sulfur dioxide in tail gas and reduce air pollution.
Owner:贵州威顿催化技术有限公司
Brine and method for producing same
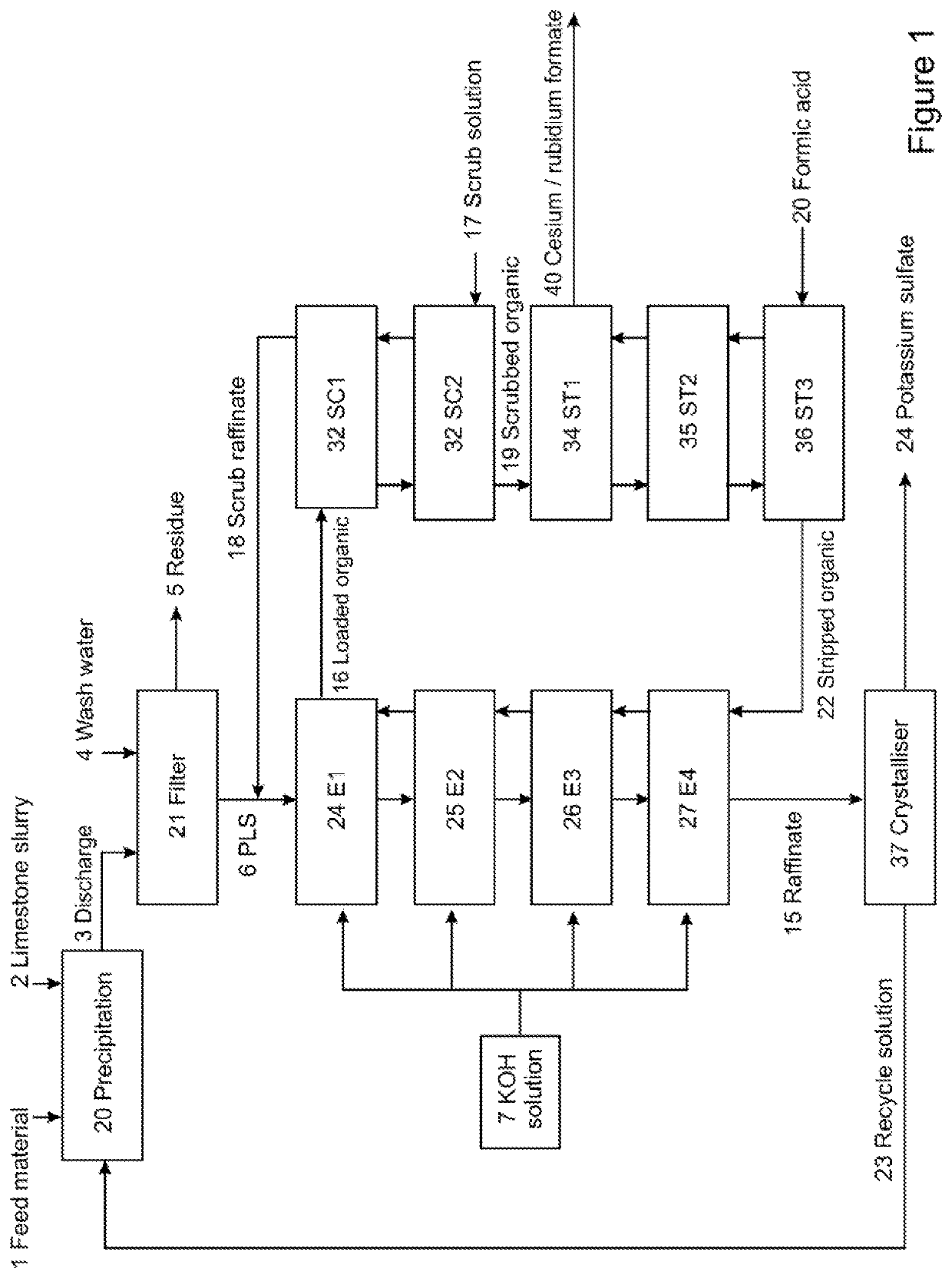
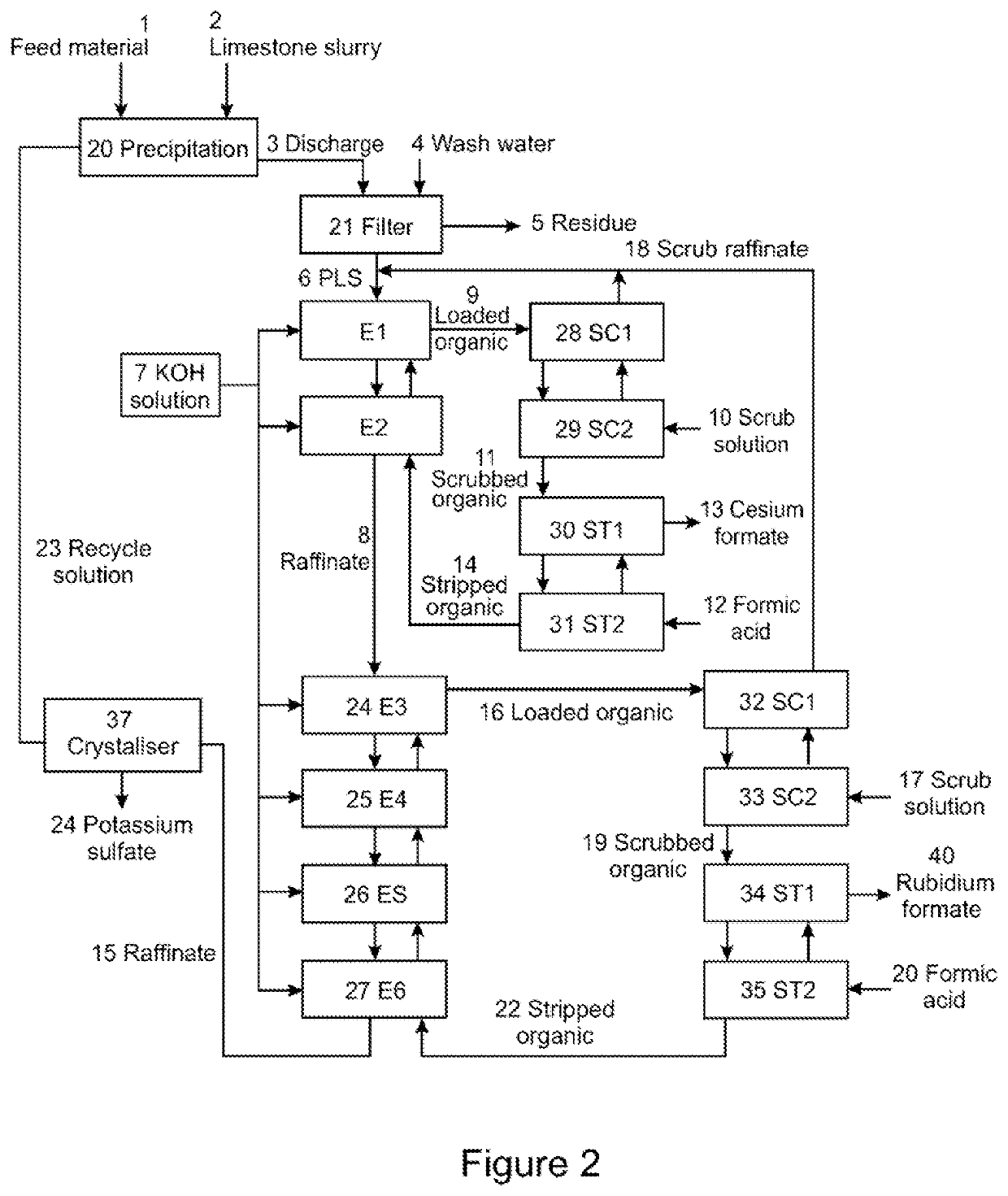
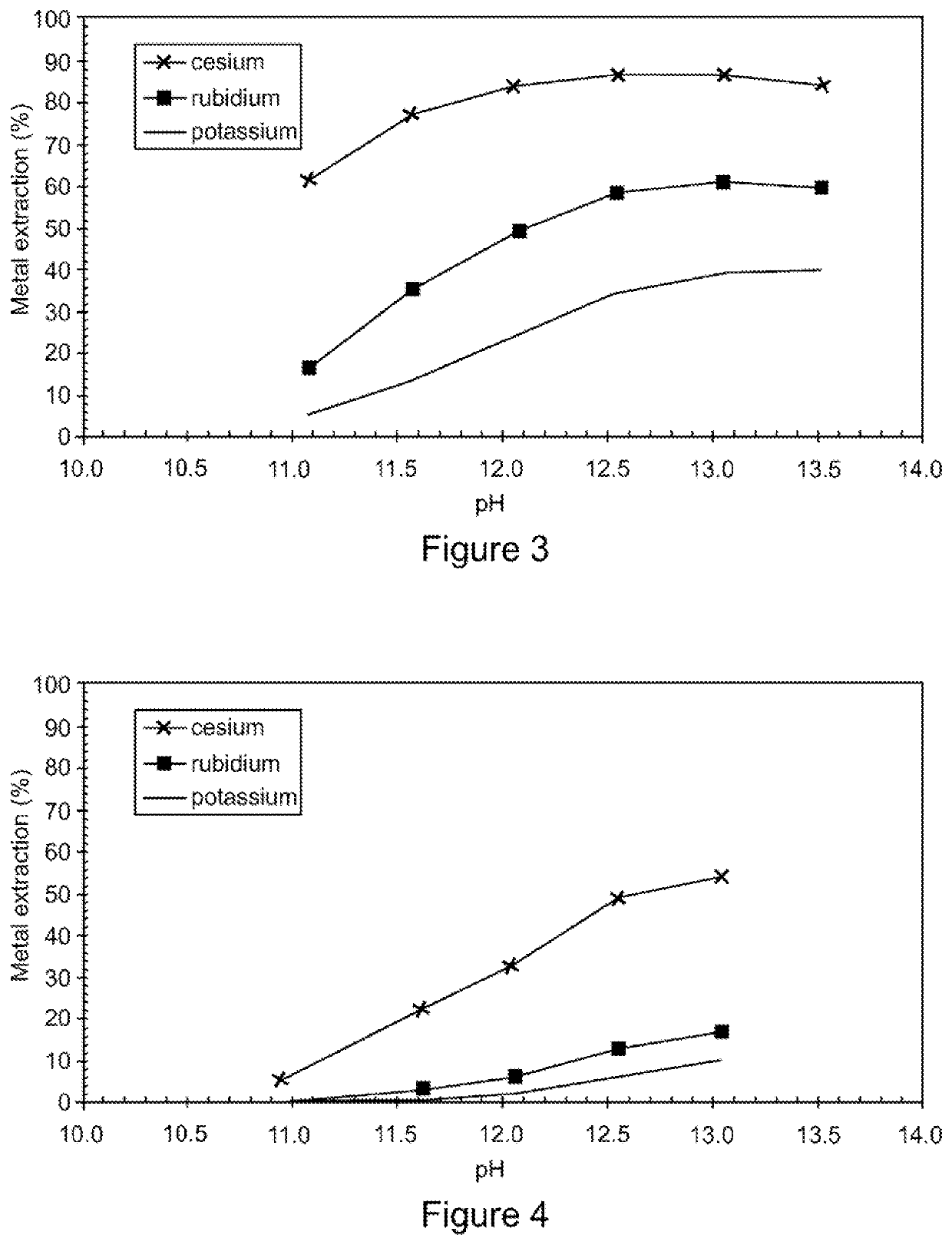
PendingUS20220056556A1Improve extraction efficiencyEfficient scrubbingCounter-current extractionLiquid solutions solvent extractionRubidium sulfateAluminium hydroxide
A method to produce a brine from mixed alum salts, the method comprising the steps of: (i) Dissolving or pulping alum salts (1) containing rubidium alum, cesium alum and / or potassium alum in water or a recycled liquor and adding a neutralising agent to precipitate (20) aluminium as aluminium hydroxide and some sulfate; (ii) Passing the product of step (i) to a solid liquid separation stage (21) to remove precipitated solids (5) from step (i); (iii) A decant or filtrate (6) from step (ii) is passed to a solvent extraction stage (24-27) whereby any contained cesium and rubidium is selectively extracted into the organic phase to form a loaded organic solution (16); (iv) Contacting the loaded organic solution (16) of step (iii) with a scrub solution (17), which is at a pH lower than the extraction pH, to effectively scrub co-loaded potassium from the organic phase; (v) Contacting the scrubbed organic (19) of step (iv) with formic acid (20) to strip cesium and rubidium from the organic, the stripped cesium and rubidium forming a cesium and / or rubidium sulfate brine (21); and (vi) Recycling the stripped organic (22) of step (v) to the extraction stage (24-27).
Owner:LI TECH PTY LTD
Method for preparing battery-grade lithium carbonate by taking lepidolite as raw material
InactiveCN112645363AReduce dosageRealize comprehensive utilizationLithium carbonates/bicarbonatesLithium hydroxideElectrical battery
The invention discloses a method for preparing battery-grade lithium carbonate by using lepidolite as a raw material. The method comprises the following steps: carrying out solid-phase reaction on concentrated sulfuric acid and lepidolite at medium and low temperatures, and leaching with water to dissolve sulfuric acid alkali metal salt; respectively separating out aluminum rubidium cesium sulfate alum salt, aluminum potassium sulfate alum salt and the like in different temperature intervals by adopting a method of adding different crystallization inducers to continuously induce crystallization and remove impurities; adopting potassium hydroxide for neutralization, crystallization and impurity removal to obtain aluminum hydroxide and potassium sulfate, applying the aluminum hydroxide for neutralizing excessive sulfuric acid, and supplementing the potassium sulfate to obtain the aluminum potassium sulfate alum salt; and finally, reacting the obtained lithium hydroxide solution with carbon dioxide to prepare battery-grade lithium carbonate. According to the method, potassium hydroxide and aluminum hydroxide are used as neutralizing reagents and potassium sulfate is used as a crystallization impurity removal reagent, so that impurity ions such as calcium and magnesium are not introduced into the production process; and finally, the obtained lithium hydroxide solution reacts with carbon dioxide to prepare the battery-grade lithium carbonate, and a major breakthrough in the technology of preparing the battery-grade lithium carbonate from lepidolite through a one-step method is achieved.
Owner:江西南氏锂电新材料有限公司
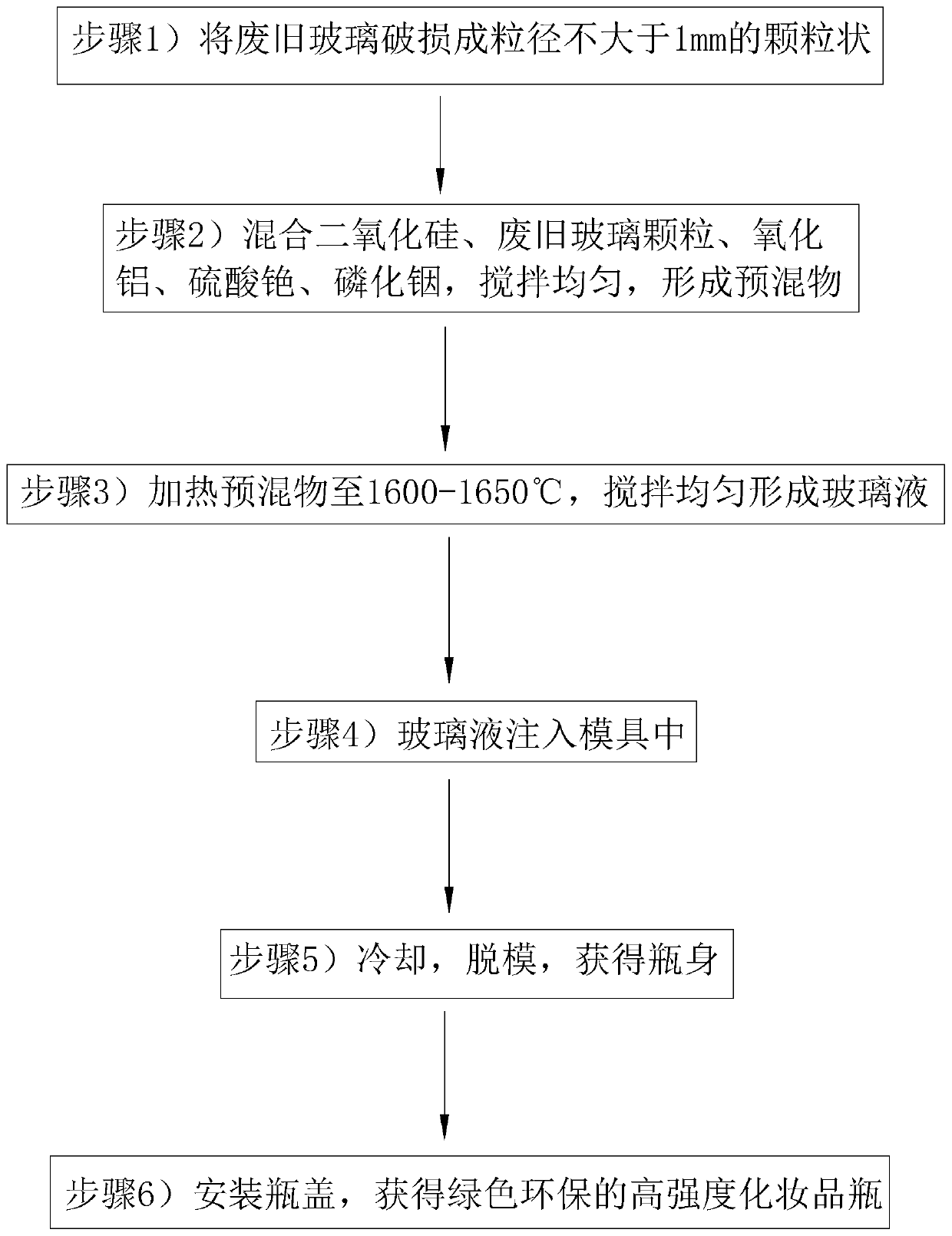
Green and environment-friendly high-strength cosmetic glass bottle
InactiveCN111362574AReduce pollutionCounteracts the negative effects of impact resistanceGlass shaping apparatusPackaging toiletriesPolymer scienceCesium sulfate
The invention relates to the field of cosmetic glass bottles, and concretely relates to a green and environment-friendly high-strength cosmetic glass bottle. The bottle comprises a bottle body, a bottle opening is formed in one end of the bottle body, and a bottle cap is detachably connected to the bottle opening; the bottle body is made of green and environment-friendly high-strength glass; and the green and environment-friendly high-strength glass comprises, by mass, 100 parts of silicon dioxide, 20 to 30 parts of waste glass, 10 to 12 parts of aluminum oxide, 1 to 2 parts of cesium sulfateand 0.3 to 0.5 part of indium phosphide. The cosmetic glass bottle has the following advantages: the cosmetic glass bottle is not prone to damage due to impact, cosmetics can be frequently carried andare not prone to damage after colliding with one another in the carrying process, and the cosmetic glass bottle is deeply loved by consumers.
Owner:广州圣威化妆品包装有限公司
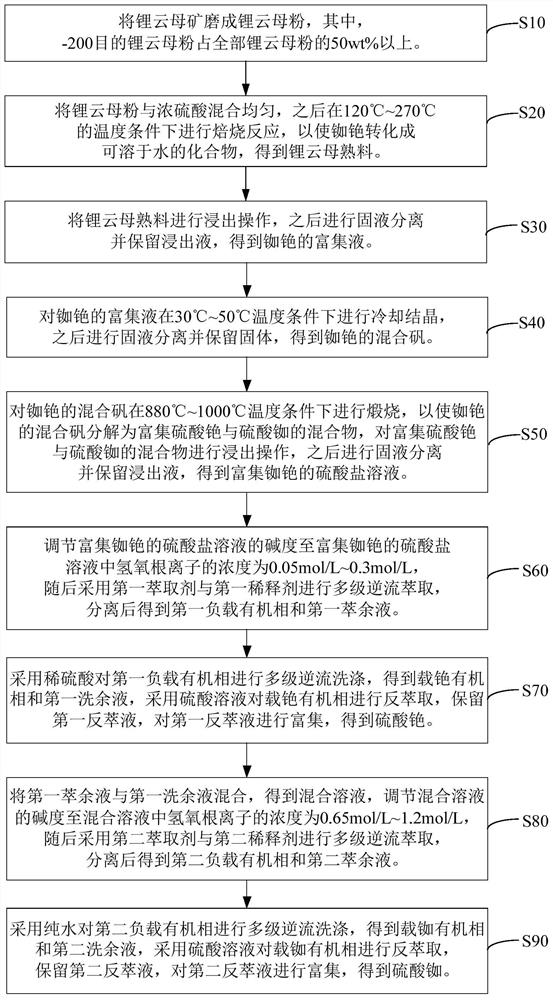
Method for extracting cesium and rubidium from lepidolite
The invention relates to a method for extracting cesium and rubidium from lepidolite. According to the method for extracting cesium and rubidium from lepidolite, separation, enrichment, purification and recovery of rubidium and cesium are effectively achieved, and the resource utilization rate is high. As verified by tests, through treatment of lepidolite ore with the Rb2O content being about 1.0%and the Cs2O content being about 0.2% by the method, sulfate solutions with the Rb content being 12 g / L and the Cs content being 2 g / L respectively can be obtained through extraction, then cesium sulfate and rubidium sulfate with the purity being 98% or above are obtained through recovery, and the total recovery rate of cesium and rubidium is 80% or above.
Owner:ZHENGZHOU MINERALS COMPOSITIVE UTILIZATION RES INST CHINESE GEOLOGICAL ACAD
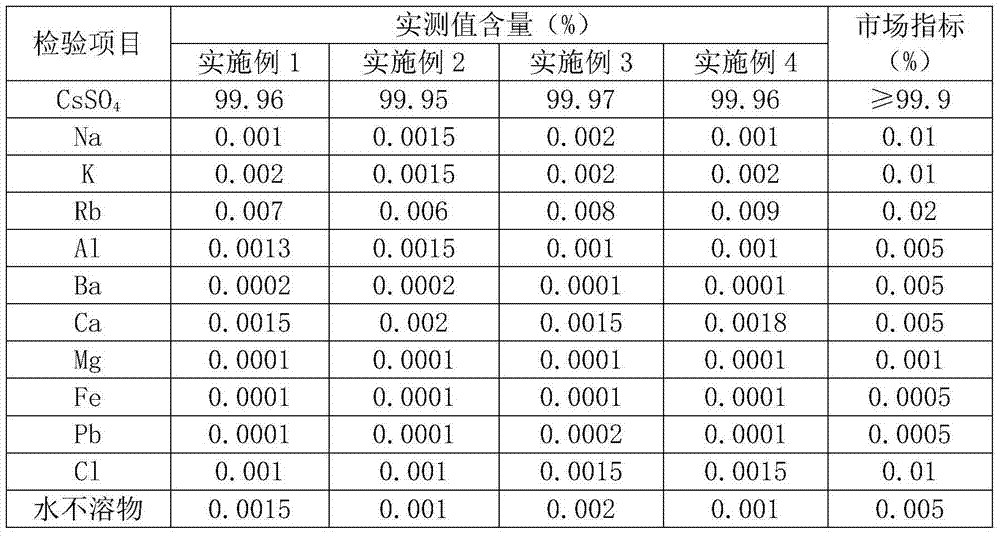
A kind of preparation method of high-purity cesium sulfate
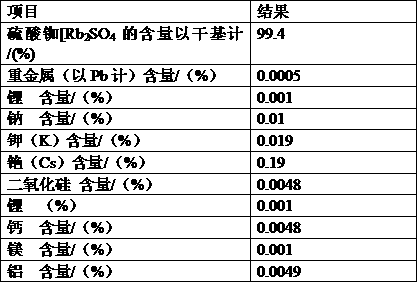
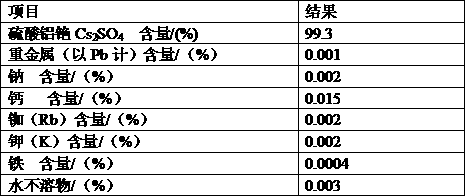
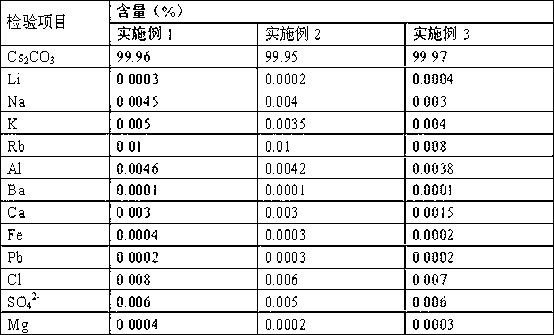
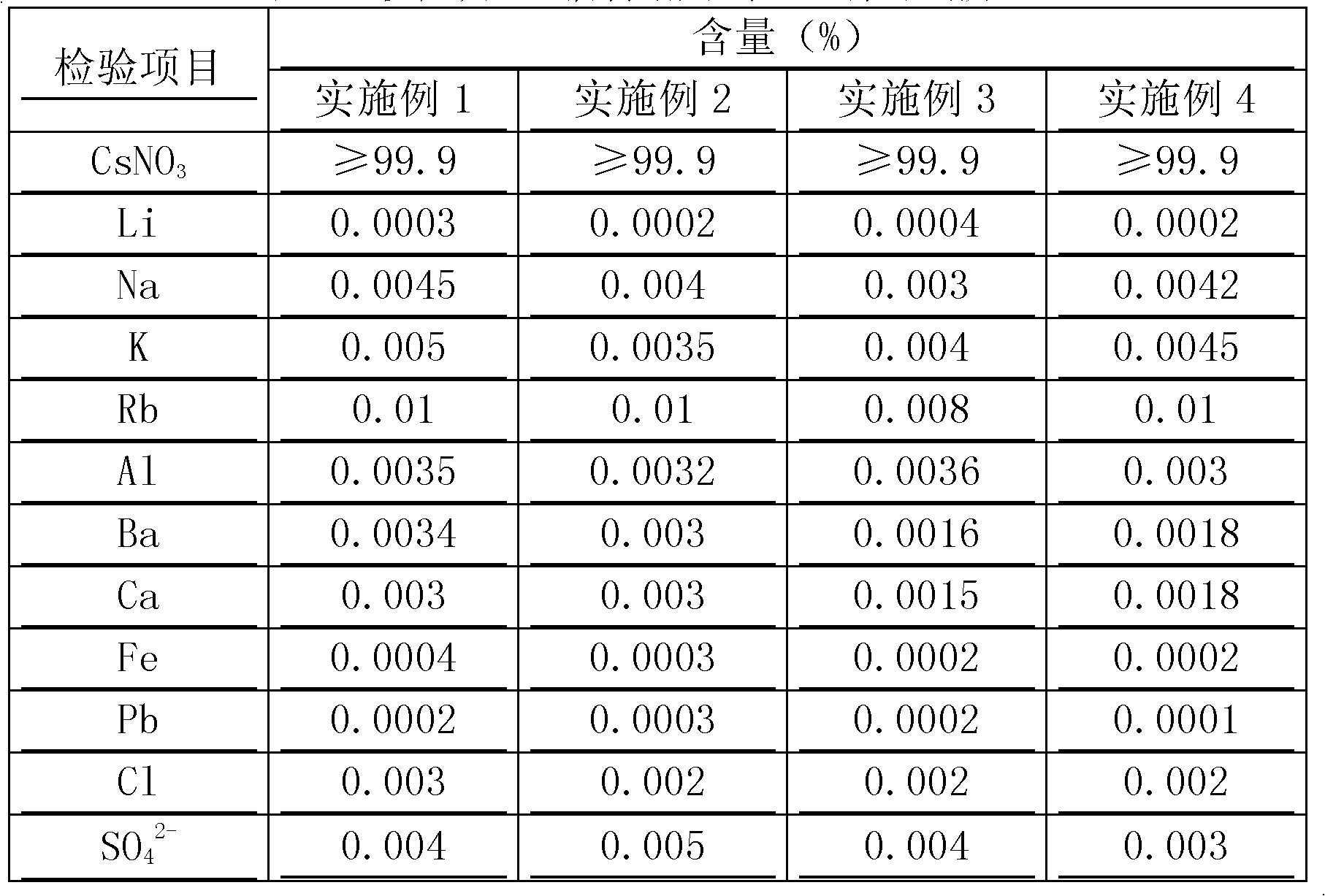
ActiveCN103803589BSimple production processSuitable for industrial productionRubidium/caesium/francium compoundsInorganic compoundTwo step
The invention belongs to the technical field of preparation methods of inorganic compound caesium sulfate, and particularly discloses a preparation method of high-purity caesium sulfate. The preparation method comprises the following steps: processing pollucite through acid leaching, vitriol sedimentation, re-crystallization and calcium oxide conversion to obtain a caesium sulfate conversion liquid, removing the impurities of the caesium sulfate conversion liquid by virtue of oxalic acid to obtain a caesium sulfate dilute solution, and finally evaporating, crystallizing and drying the caesium sulfate dilute solution to obtain the caesium sulfate finished product. The production process is simple in flow, and the preparation method is suitable for the industrial production; the raw material pollucite is a main raw material for producing the cesium salt, the market source is wide, and the raw material is easy to obtain; by comprising two-step impurity removal, the process is easier to control, the operation requirement is lowered, and the product quality stability is greatly improved.
Owner:HUBEI BAIJIERUI ADVANCED MATERIALS
A cesium magnesium sulfate nonlinear optical crystal and its preparation method and application
ActiveCN110079858BShort UV cut-off edgeModerate multiplier conversion efficiencyPolycrystalline material growthFrom frozen solutionsNonlinear optical crystalCrystal system
The invention discloses a Cs2Mg3(SO4)4 non-linear optical crystal as well as a preparation method and application thereof. The preparation method comprises the following step: by taking Cs2SO4 and Mg-containing sulfate as raw materials, sintering so as to obtain a Cs2Mg3(SO4)4 microcrystal; or mixing and melting Cs2SO4 and Mg-containing sulfate, thereby obtaining the Cs2Mg3(SO4)4 non-linear optical crystal. The Cs2Mg3(SO4)4 disclosed by the invention belongs to an orthorhombic system P212121 space group, a MgO6 polyhedron and a SO4 tetrahedron in the structure are in co-top point connection toform a three-dimensional frame, and in addition, frame pores are filled with Cs<+> ions. By adopting the crystal, a pure phase and a single crystal of Cs2Mg3(SO4)4 are obtained, the synthesis methodis simple, the yield is high, and the crystal has a short ultraviolet cut-off side, a moderate frequency doubling conversion efficiency and good growth habits, and can be applied to non-linear opticaldevices such as frequency conversion, optical parameter oscillation, electro-optical modulation and communication.
Owner:MINJIANG UNIV
Methods to recover cesium formate from a mixed alkali metal formate blend
ActiveUS9452966B2Efficient and inexpensivePreparation from carboxylic acid saltsOrganic compound preparationSodium bicarbonateFormate
Methods to recover or separate cesium formate or rubidium formate or both from a mixed alkali metal formate blend are described. One method involves adding cesium sulfate or rubidium sulfate to the mixed alkali metal formate blend in order to preferentially precipitate potassium sulfate from the mixed alkali metal formate blend. Another method involves adding cesium carbonate or cesium bicarbonate or both to preferentially precipitate potassium carbonate / bicarbonate and / or other non-cesium or non-rubidium metals from the mixed alkali metal blend. Further optional steps are also described. Still one other method involves converting cesium sulfate to cesium hydroxide.
Owner:CABOT SPECIALTY FLUIDS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com