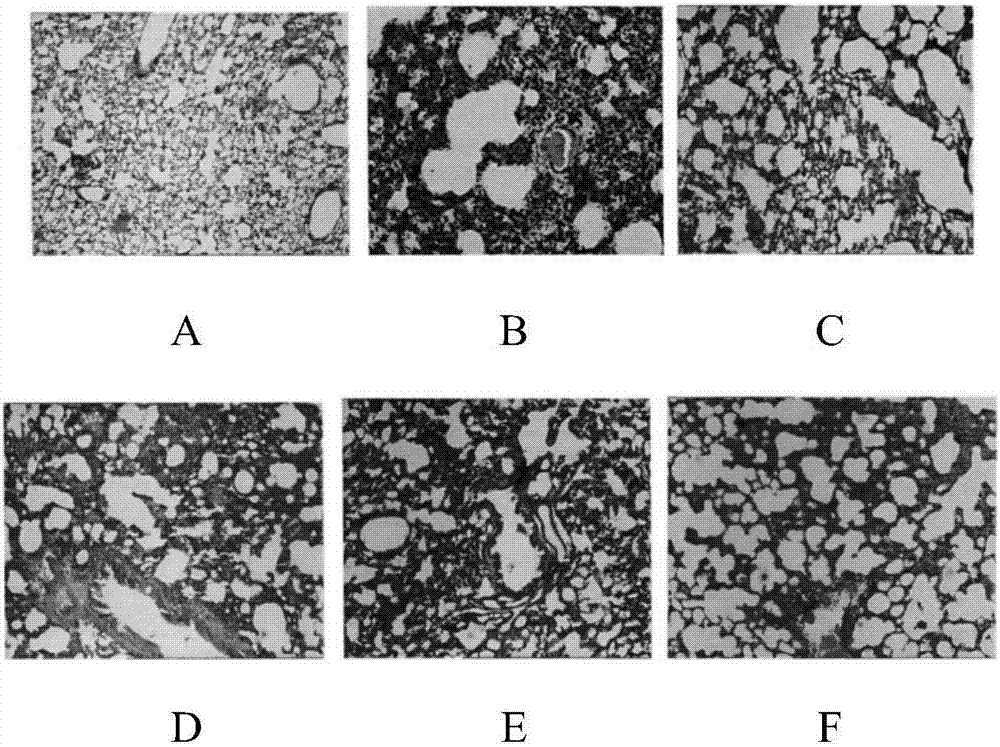
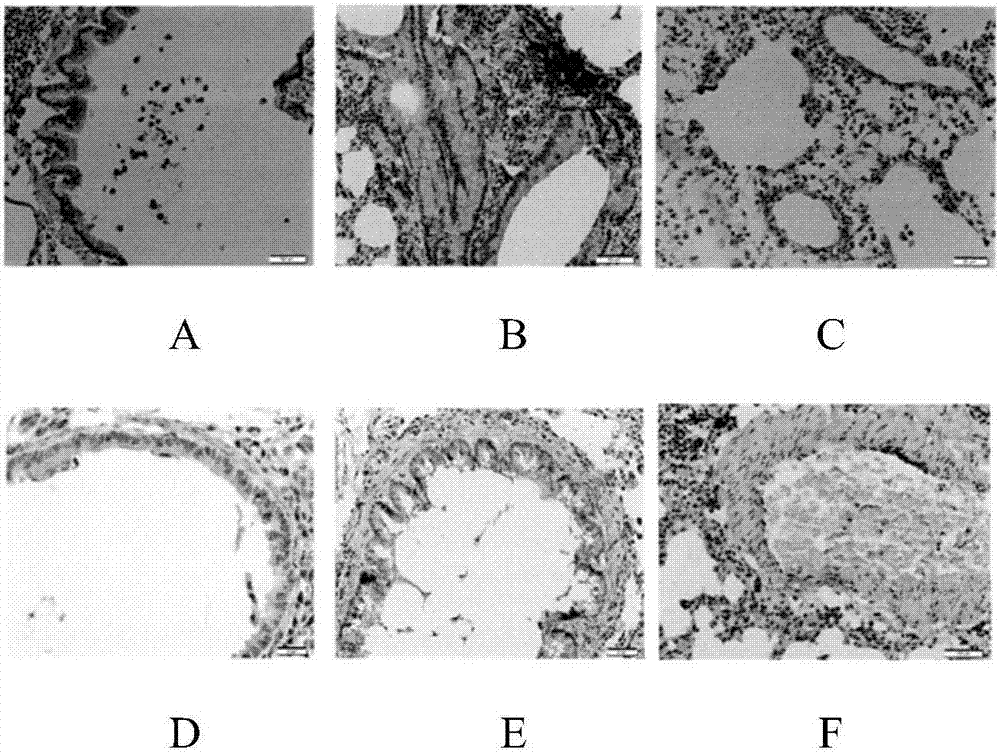
Pharmaceutical composition for treating asthma
A composition and drug technology, applied in the field of medicine, can solve the problems of reduced drug efficacy, inability to cure asthma, low immunity, etc., and achieve the effects of obvious effects, improvement of lung tissue lesions, and reduction of levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
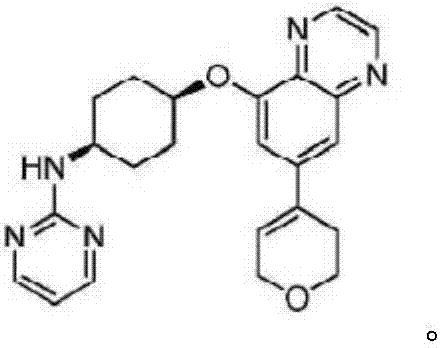
[0017] The characterization of experimental example 1 medicine of the present invention
[0018]
[0019] 1 H-NMR (300MHz, CDCl 3 )δ8.94-8.76 (m, 2H), 8.29 (d, J = 4.8Hz, 2H), 7.67 (d, J = 1.7Hz, 1H), 6.53 (t, J = 4.8Hz, 1H), 6.37 ( tt,J=3.1,1.5Hz,1H),5.30(d,J=7.9Hz,1H),4.87(dt,J=7.5,3.6Hz,1H),4.43(q,J=2.8Hz,2H), 4.02(t, J=5.5Hz, 3H), 2.68(dqd, J=6.0, 3.4, 3.0, 1.8Hz, 2H), 2.35-2.11(m, 2H), 2.07-1.84(m, 6H); ESMS( M+H + ) = 404.2.
experiment example 2
[0020] The effect of experimental example 2 medicine treatment asthma of the present invention
[0021] Male SD rats aged 6-8 weeks, SPF grade, and weighing 200±20 g were divided into a normal group of 10 and a model group according to the random number table method. The above-mentioned rats were kept in the same room and divided into cages, 5 in each cage, fed under sterile conditions, and had free access to drinking water.
[0022] Animal grouping and model preparation
[0023] Rats except the normal group were established with asthma model: on the 1st day and the 8th day, 1ml of antigen solution (containing ovalbumin 100mg, aluminum hydroxide dry powder 200mg, inactivated Bacillus pertussis 5×10 9 One, dissolved in 1ml of normal saline), on the 15th day, nebulized inhalation of 30ml of 5% ovalbumin prepared with normal saline was stimulated, once a day, 30min each time, for 14 days in a row.
[0024] Normal group: On the 1st day and the 8th day, 1ml of normal saline was i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com