Compound for treating cerebral infarction and preparation method thereof
A compound and alkyl technology, which is applied in the field of preparation of drugs for the treatment of cerebral infarction and compounds for the treatment of cerebral infarction, can solve the problems of stealing blood, small clinical curative effect, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
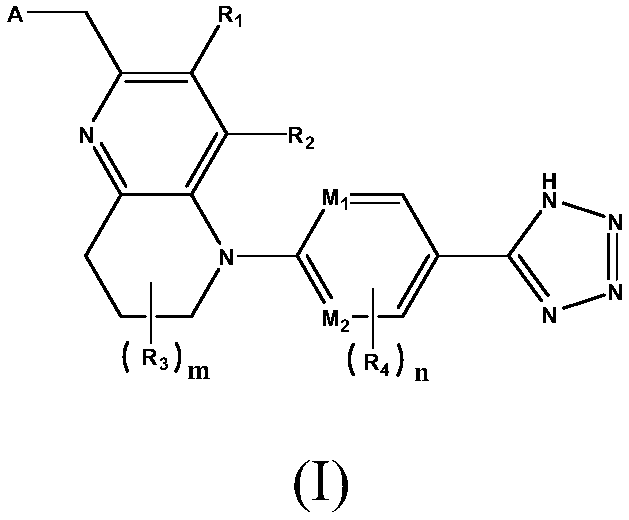
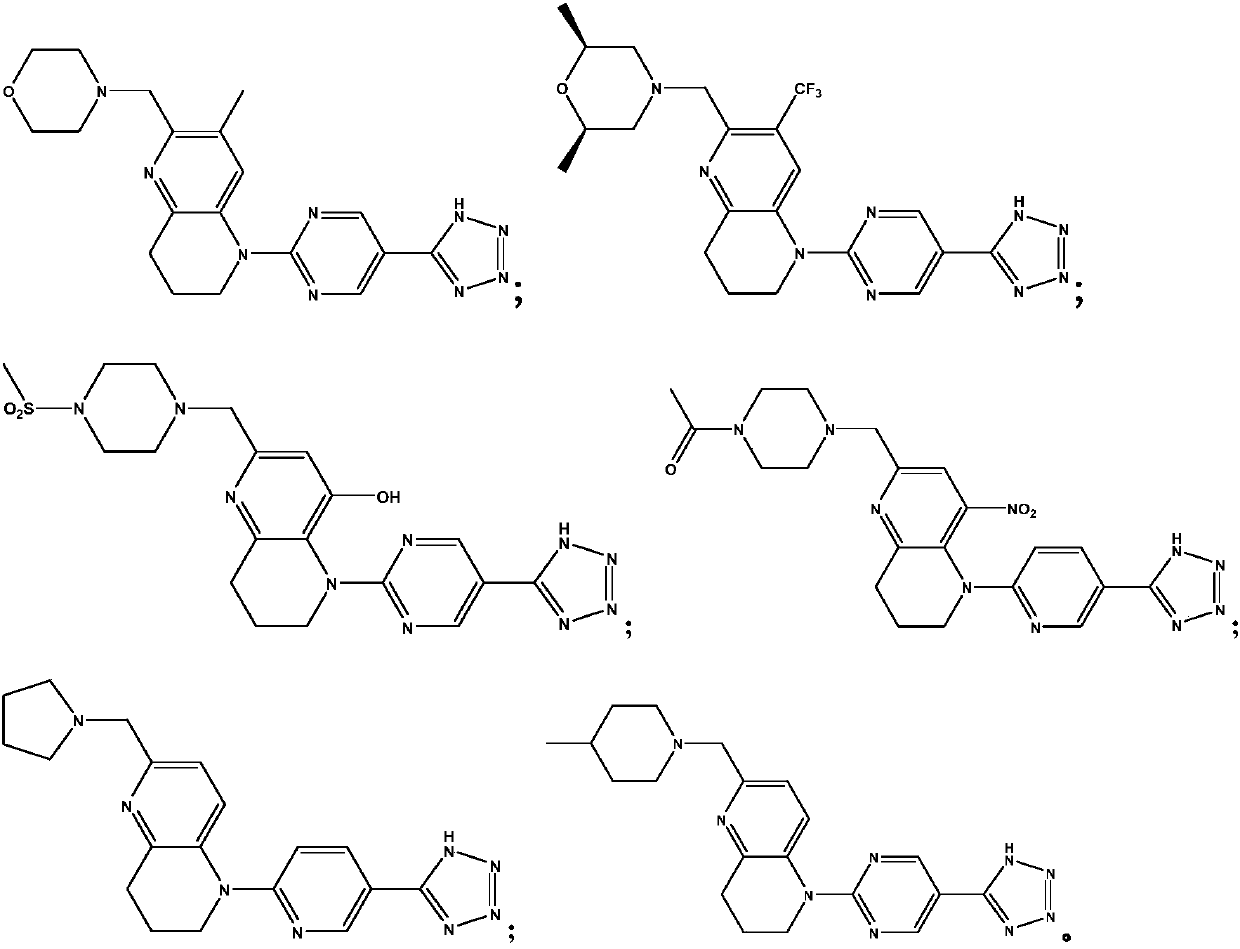
[0063] 4-((5-(5-(1H-tetrazol-5-yl)pyrimidin-2-yl)-3-methyl-5,6,7,8-tetrahydro-1,5-naphthyridine -2-yl)methyl)morpholine
[0064]
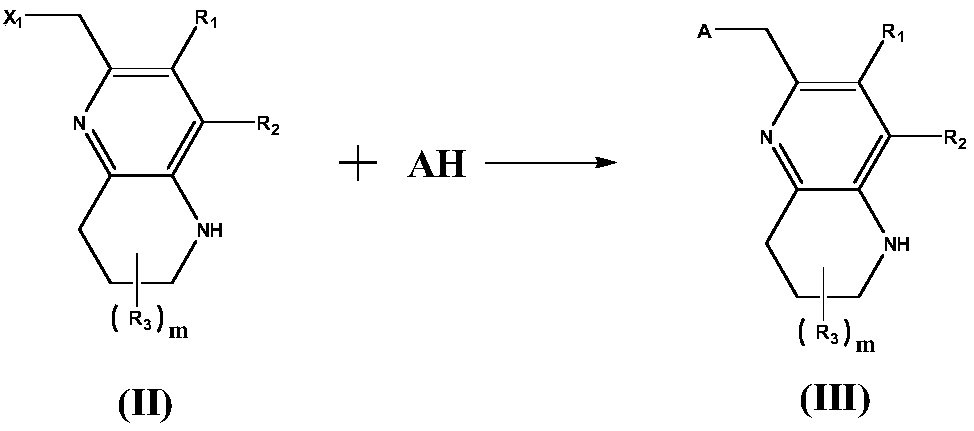
[0065] Step 1: Dissolve morpholine (2.58 g, 30 mmol) and 60% NaOH (1.40 g, 35 mmol) in 150 mL of anhydrous THF, and stir at 50° C. for 30 min under nitrogen protection. Cool to room temperature, slowly add dropwise 100mL of anhydrous THF solution containing 6-bromomethyl-7-methyl-1,2,3,4-tetrahydro-1,5-naphthyridine (6.01g, 25mmol) Then heat and stir for 3h, pour the reaction solution into 150mL of ice water, extract three times with ethyl acetate, combine the organic phases, wash once with saturated brine, dry the organic phases with anhydrous magnesium sulfate, filter, and evaporate the solvent under reduced pressure. The resulting residue was subjected to column chromatography (eluent volume ratio: cyclohexane:ethyl acetate=6:1), and the solvent was distilled off under reduced pressure to obtain off-white solid 4-((3-methyl-5,6,7, 8-Tetrahy...
Embodiment 2
[0072] (2S,6R)-4-((5-(5-(1H-tetrazol-5-yl)pyrimidin-2-yl)-3-trifluoromethyl-5,6,7,8-tetrahydro- 1,5-naphthyridine-2-yl)methyl)-2,6-dimethylmorpholine
[0073]
[0074] According to the method of Example 1, (2S,6R)-2,6-dimethylmorpholine was used instead of morpholine, and 6-bromomethyl-7-trifluoromethyl-1,2,3,4-tetrafluoromethyl-1,2,3,4-tetra Hydrogen-1,5-naphthyridine replaced 6-bromomethyl-7-methyl-1,2,3,4-tetrahydro-1,5-naphthyridine, and the total yield of the three steps was 48.7%.
[0075] ESI-MS: 475.21[M+H] +
[0076] Elemental analysis: theoretical value / measured value, C(53.05 / 53.17), H(5.09 / 5.02), F(11.99 / 11.89), N(26.51 / 26.54), O(3.36 / 3.38)
[0077] 1 H NMR (400MHz, CDCl 3 )δ13.09(s,1H),9.06(s,2H),7.35(s,1H),3.94(s,2H),3.51(m,2H),3.08(t,2H),3.02(t,2H ), 2.48(d,4H), 1.92(m,2H), 1.18(d,6H).
Embodiment 3
[0079] 5-(5-(1H-tetrazol-5-yl)pyridin-2-yl)-2-((4-methylsulfonyl-piperazin-1-yl)methyl)-5,6,7, 8-tetrahydro-1,5-naphthyridine-4-ol
[0080]
[0081] According to the method of Example 1, morpholine was replaced with 1-methylsulfonyl-piperazine, and 2-bromomethyl-5,6,7,8-tetrahydro-1,5-naphthalene-4- Alcohol was used instead of 6-bromomethyl-7-methyl-1,2,3,4-tetrahydro-1,5-naphthyridine, and the total yield of the three steps was 45.5%.
[0082] ESI-MS: 472.18[M+H] +
[0083] Elemental analysis: theoretical value / measured value, C(50.94 / 50.82), H(5.34 / 5.42), N(26.73 / 26.64), O(10.18 / 10.27), S(6.80 / 6.85)
[0084] 1 H NMR (400MHz, CDCl 3 )δ13.09(s,1H),10.04(s,1H),8.35(d,1H),7.62(q,1H),6.74(d,1H),6.48(s,1H),3.94(s,2H ), 3.08(t,2H), 3.02(t,2H), 2.93(s,3H), 2.43(t,4H), 2.33(t,4H), 1.92(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com