Tadalafil solid dispersion system and preparation method thereof
A tadalafil, solid dispersion technology, applied in respiratory system diseases, urinary system diseases, cardiovascular system diseases, etc., can solve problems such as poor effect, achieve good reproducibility, simple process operation, and improve bioavailability The effect of degree and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
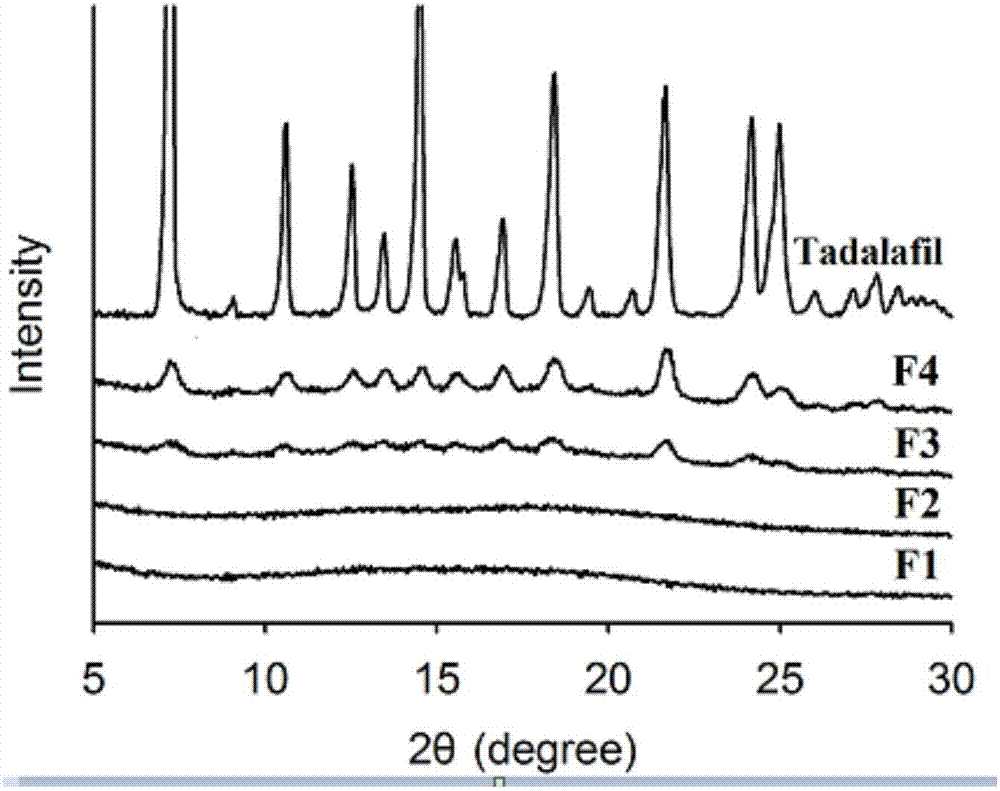
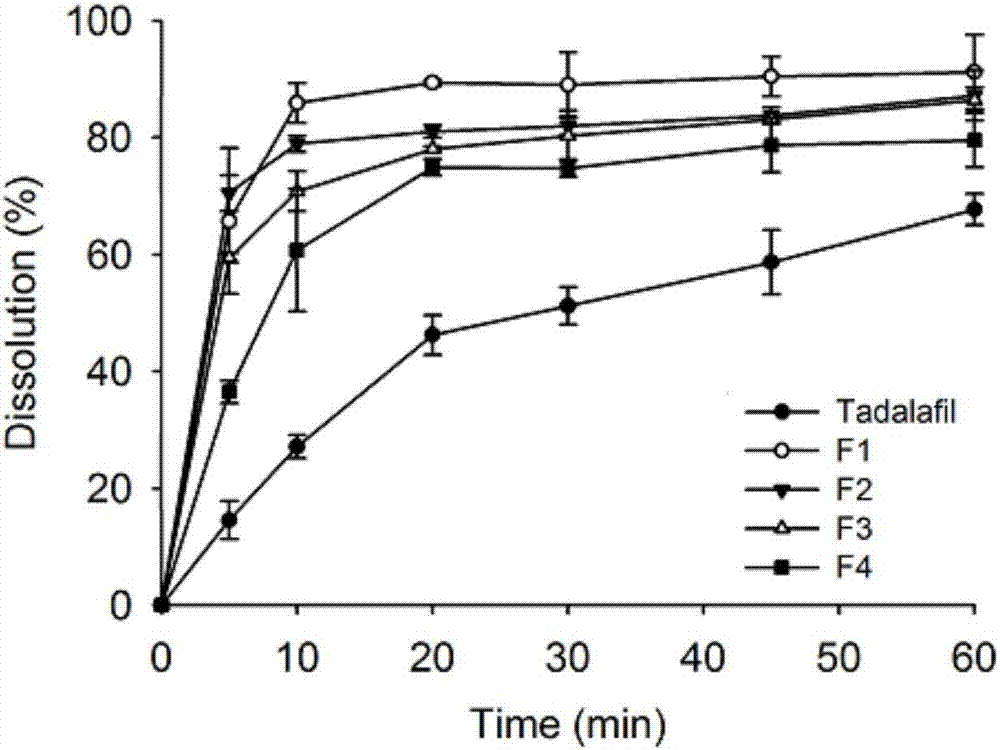
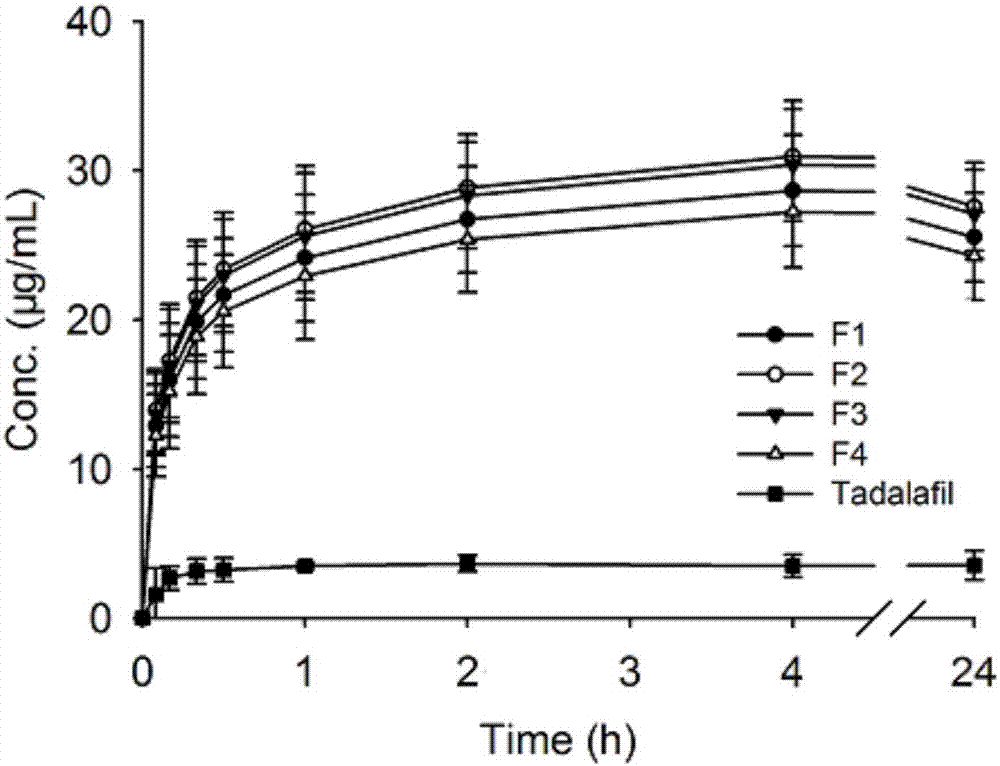
[0044] This embodiment provides a tadalafil solid dispersion system (F1), which is mainly made of tadalafil and soluplus, wherein, by weight g, tadalafil is 1 g, and soluplus is 9 g.
[0045] This embodiment provides also a kind of method for preparing tadalafil solid dispersion system:
[0046] Acetone and water were mixed at a volume ratio of 9:1, and then the tadalafil raw material was added so that the content of the tadalafil raw material in the mixed solvent was 1% (W / V). The inlet temperature of spray drying is 85°C, the outlet temperature is 30°C, and the hot air volume is 35m 3 / h, peristaltic pump flow rate 2mL / min, atomizing gas flow rate 100L / h.
[0047] After fully mixing 1 g of tadalafil and 9 g of soluplus uniformly, the mixed mixture is added into a hot-melt extruder for heating, melting and extrusion. Specifically, the mixture was melted at a temperature of 180° C., stirred at a rotation speed of 50 rpm, and extruded at a speed of 1.2 g / min at a temperature ...
Embodiment 2
[0049] This embodiment provides a tadalafil solid dispersion system (F2), which is mainly made of tadalafil and soluplus, wherein, in terms of weight g, tadalafil is 2 g, and soluplus is 8 g.
[0050] This embodiment provides also a kind of method for preparing tadalafil solid dispersion system:
[0051] Acetone and water were mixed at a ratio of 8:1 by volume, and then a mixture of 2 g of tadalafil and 8 g of soluplus was added, and the content of the mixture in the mixed solvent was 1.5% (W / V). The inlet temperature of spray drying is 95°C, the outlet temperature is 30°C, and the hot air volume is 33.5m 3 / h, peristaltic pump flow rate 2.5mL / min, atomizing gas flow rate 400L / h.
[0052] The spray-dried mixture is added to a hot-melt extruder for heat-melting and extrusion. Specifically, after melting the mixture at a temperature of 160° C. and stirring at a rotation speed of 100 rpm, the mixture is extruded at a temperature of 130° C. at a speed of 0.5 g / min. The extruded...
Embodiment 3
[0054] This embodiment provides a tadalafil solid dispersion system (F3), which is mainly made of tadalafil and soluplus, wherein, in terms of weight g, tadalafil is 3 g, and soluplus is 7 g.
[0055] This embodiment provides also a kind of method for preparing tadalafil solid dispersion system:
[0056] Acetone and water were mixed at a volume ratio of 8.5:1, and then a mixture of 3 g of tadalafil and 7 g of soluplus was added, and the content of the mixture in the mixed solvent was 2% (W / V). The inlet temperature of spray drying is 80°C, the outlet temperature is 60°C, and the hot air volume is 15m 3 / h, peristaltic pump flow rate 3mL / min, atomizing gas flow rate 500L / h.
[0057] The spray-dried mixture is added into a hot-melt extruder to be heated, melted and extruded. Specifically, after melting the mixture at a temperature of 190° C. and stirring at a rotation speed of 70 rpm, the mixture is extruded at a temperature of 130° C. at a speed of 15 g / min. The extruded mix...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com