Crystal forms, salts and complexes of dihydropyrimidine derivatives and their application in medicine
A compound and crystal form technology, which is applied in the directions of drug combinations, medical preparations containing active ingredients, antitumor drugs, etc., can solve the problems of inconvenient development of preparations, unfavorable storage and weighing, and unsatisfactory stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
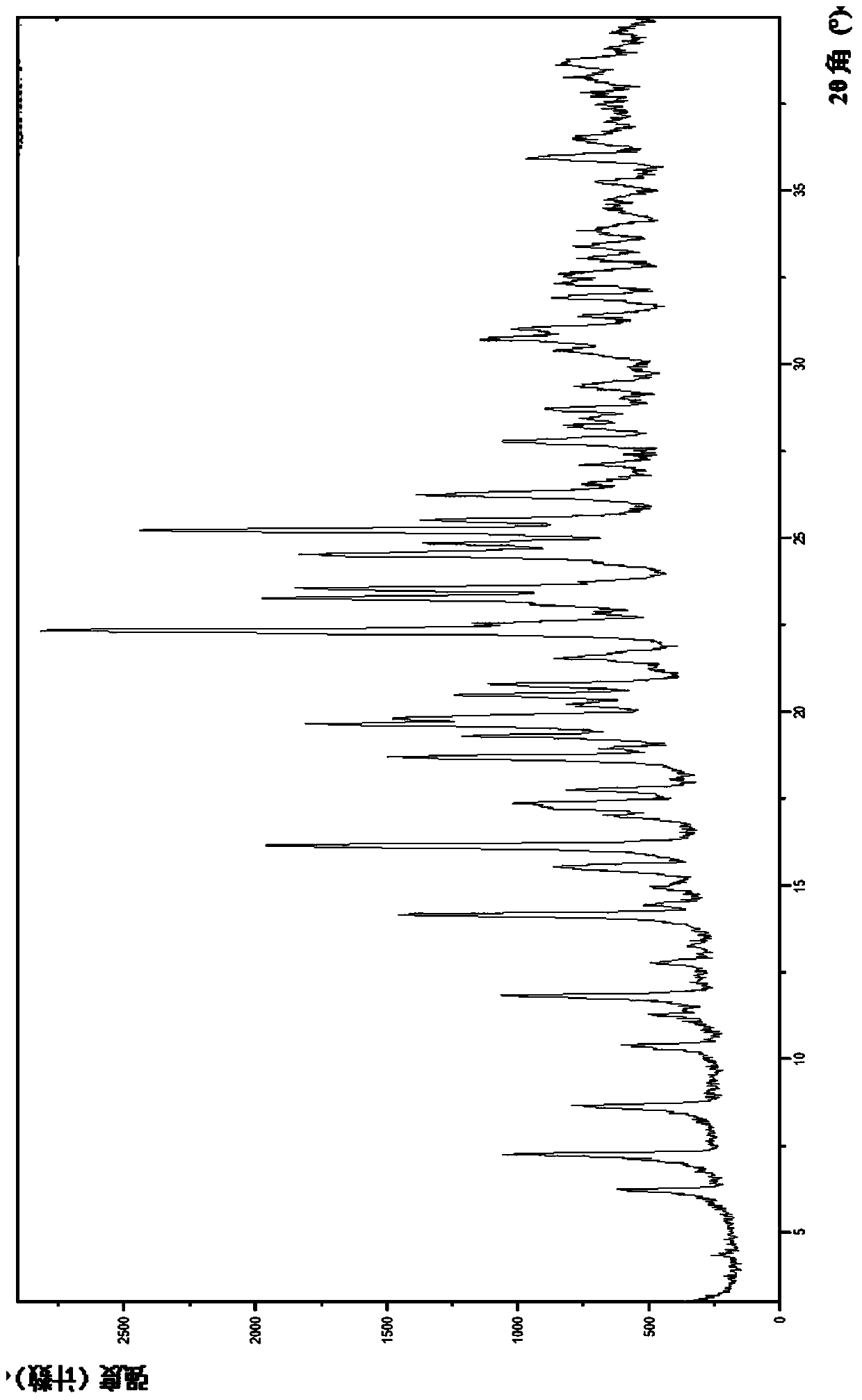
[0153] 1. Preparation of the crystal form I(A) of the citrate of the compound shown in formula (I) or the citric acid complex
[0154] Add 3-((R)-4-(((R)-6-(2-bromo-4-fluorophenyl)-5-(ethoxycarbonyl)-2-(thiazole- 2-yl)-3,6-dihydropyrimidin-4-yl)methyl)morpholin-2-yl)propionic acid (I) (50g, 86mmol) and ethyl acetate (300mL), after stirring and dissolving completely, The temperature was raised to 45°C, and a solution of citric acid (9.9g, 51.53mmol) prepared in advance in acetone (100mL) was added. After the addition, the residual solution was rinsed with acetone (50mL), and then the temperature was raised to 50°C and kept stirring for 1.5 hours. Heating was stopped, the temperature was naturally cooled to room temperature, and stirring was continued for 24 hours. Solids were precipitated, filtered, and the filter cake was washed with ethyl acetate (250 mL). The filter cake was collected, air dried for 1 hour, then vacuum dried at room temperature for 3 hours, followed by vacu...
Embodiment 2
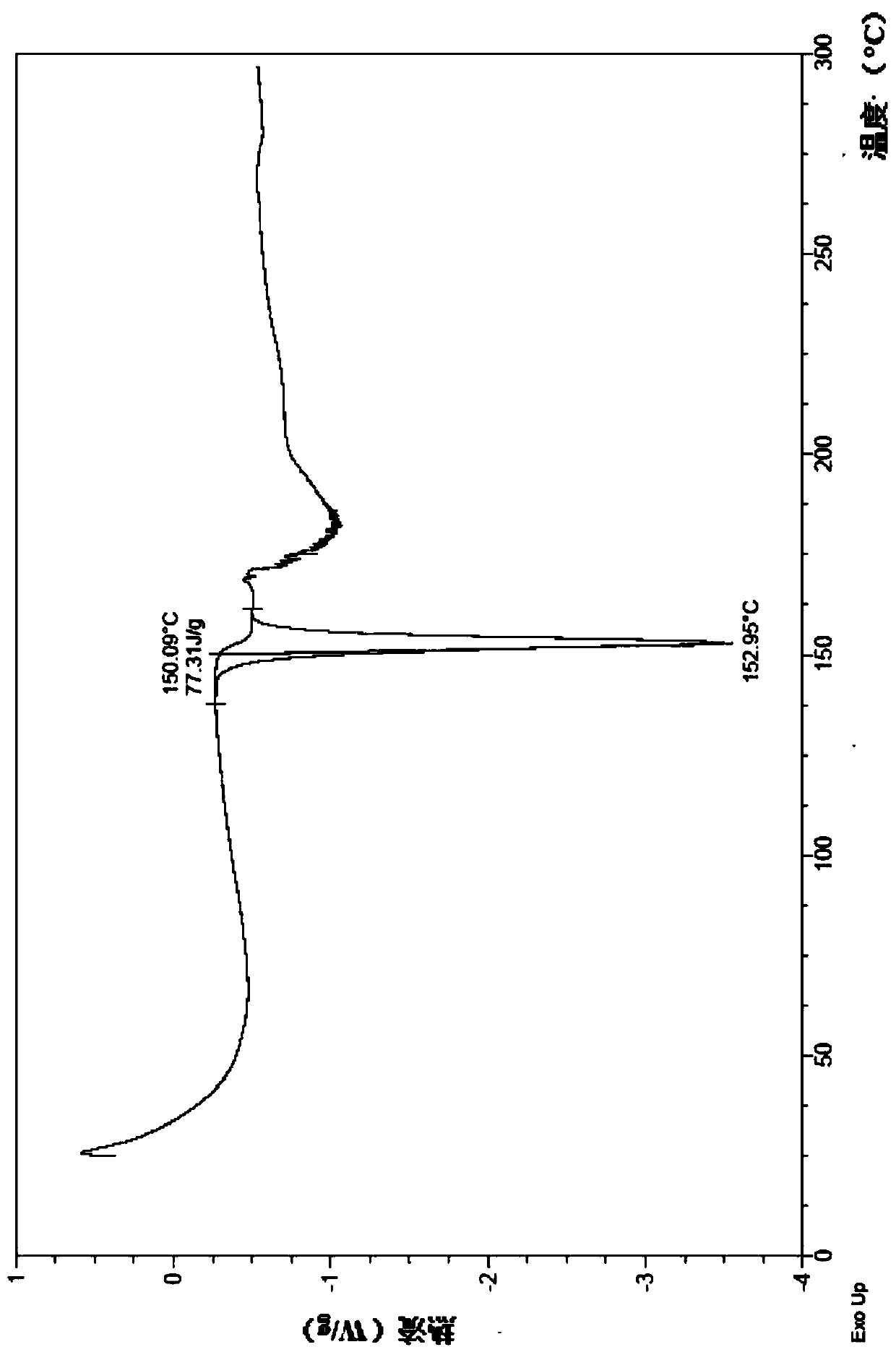
[0168] 1. Preparation of the mesylate crystal form I(B) of the compound represented by formula (I)
[0169] Add 3-((R)-4-(((R)-6-(2-bromo-4-fluorophenyl)-5-(ethoxycarbonyl)-2-(thiazole-2 -yl)-3,6-dihydropyrimidin-4-yl)methyl)morpholin-2-yl)propionic acid (I) (10g, 17.20mmol), dichloromethane (84mL) and ethyl acetate (50mL ), stirred at room temperature until the solution was completely dissolved, and added dropwise a solution of methanesulfonic acid (1.82 g, 18.9 mmol) in ethyl acetate (6 mL). After dropping, the resulting reaction solution was stirred at room temperature for 12 hours, and solids were precipitated, filtered, and the filter cake was washed with ethyl acetate (50 mL). The filter cake was collected and dried under vacuum at room temperature for 6 hours to obtain the product as a yellow solid (8 g, yield 68.64%).
[0170] 1 H NMR (400MHz,D 2 O)δ(ppm):7.86(d,1H),7.77(d,1H),7.42-7.38(m,2H),7.06(td,1H),6.11(s,1H),4.66(d,1H) ,4.43(d,1H),4.16-4.12(m,1H),4.04-3.92(...
Embodiment 3
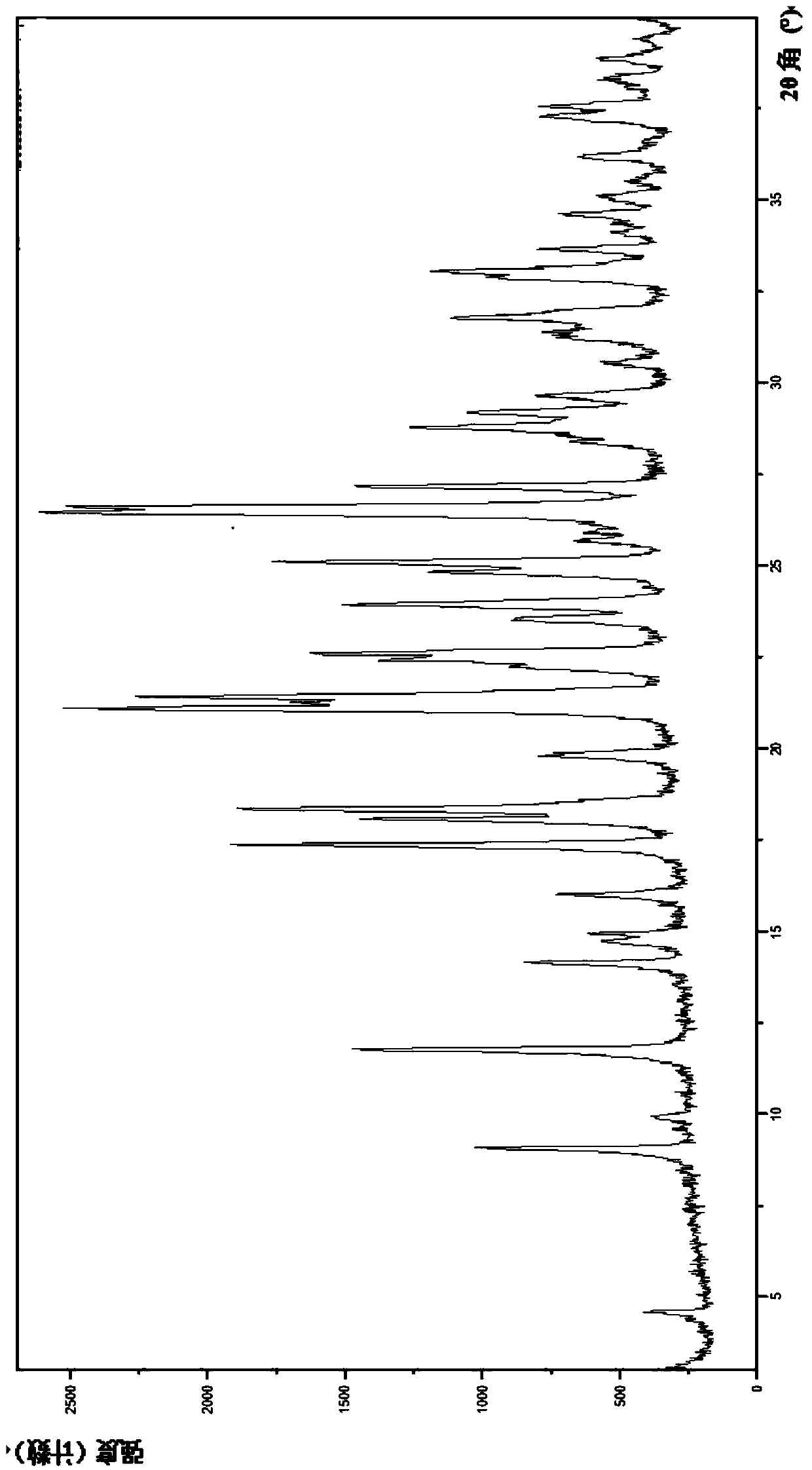
[0179] 1. Preparation of the mesylate crystal form II of the compound represented by formula (I)
[0180]Add 3-((R)-4-(((R)-6-(2-bromo-4-fluorophenyl)-5-(ethoxycarbonyl)-2-(thiazole- 2-yl)-3,6-dihydropyrimidin-4-yl)methyl)morpholin-2-yl)propanoic acid (I) (50 g, 86.00 mmol), acetone (720 mL) and ethyl acetate (100 mL) , heated to 55°C and stirred until completely dissolved. A solution of methanesulfonic acid (7.85 g, 81.7 mmol) in ethyl acetate (80 mL) was added dropwise thereto. After the dropwise addition was completed, the resulting reaction solution was kept warm and stirred for 1.5 hours. After cooling down to room temperature, the mixture was kept warm and stirred for 12 hours, and solids were gradually precipitated out. After filtration, the filter cake was washed with ethyl acetate (300 mL), collected and vacuum-dried at 70° C. for 8 hours to obtain the product as a yellow solid (51 g, yield 88%).
[0181] 2. Identification of the mesylate crystal form II of the co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com