Method of preparing recombinant human alpha-L-iduronidase
An iduronidase and purpose technology, applied in the field of genetic engineering, can solve the problems of lack of treatment research, high price, unfavorable development of gene therapy technology, etc., and achieve the effect of good application effect and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] rhIDUA expression plasmid construction
[0033] The IDUA coding region gene sequence (GenBank: M74715.1) was optimized and synthesized for the CHO species, and the synthesized gene sequence was recombined into the plasmid vector pUC19 to obtain a plasmid containing the target gene as pUC-IDUA. Use BglII and PacI to double digest the pCMV plasmid and recover the large fragment, then use BglII and PacI to double digest the pUC-IDUA plasmid to recover the target gene fragment. After digestion, the vector fragment with the same sticky end and the target gene will be produced. to connect. The ligation product was transformed into Top10 Escherichia coli competent cells, spread on 2YT plate medium, and stood at 37°C overnight. Pick a single colony for small-scale culture and extract the plasmid for identification.
[0034] The sequence of rhIDUA is as follows:
[0035] SEQ ID No 1
[0036]GCCACCATGAGGCCTCTGAGGCCCCGTGCTGCTCTGCTCGCCCTCCTCGCTTCCCTGCTGGCCGCTCCTCCTGTGGCTCCCGCCG...
Embodiment 2
[0038] Expression of rhIDUA in CHO DG44 cells
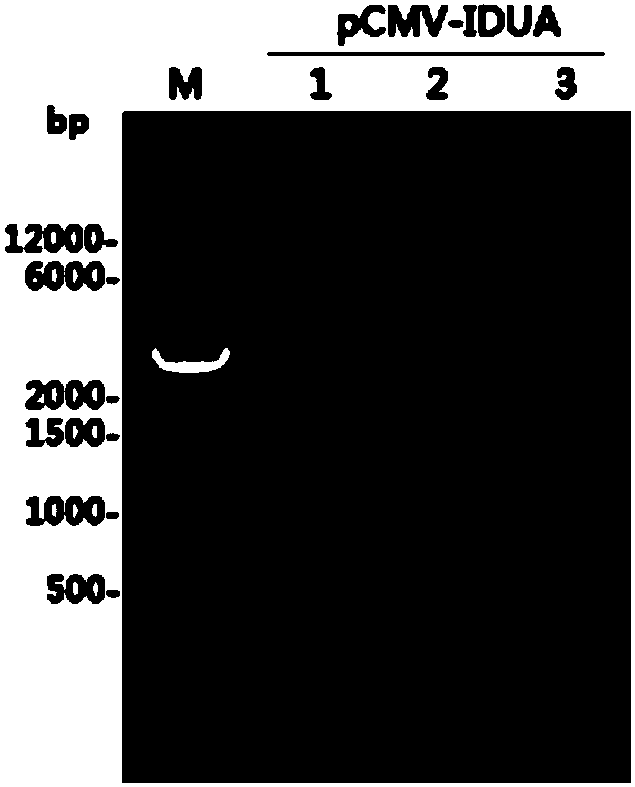
[0039] The correct recombinant plasmid pCMV-IDUA (such as figure 1 ) were transfected into CHO DG44 cells by electroporation, and the cell mixture was transferred to a 6-well plate containing SFM4 medium, cultured in a 37°C incubator, and MTX (10nM) was added after 48h for pressurized screening , when the cell viability recovered to more than 90%, MTX was increased to 100nM; the cells were added with MTX to 500nM, and after the viability recovered to more than 90%, the cells were transferred to shake flasks for culture. On the third and fifth days of culture, respectively, Glucose (4g / L) was added, and the cultivation was continued until the 7th day. The culture solution was taken and centrifuged at 5000 rpm for 15 min at 4°C, and the supernatant was collected and filtered through a 0.45 μm filter membrane for purification.
Embodiment 3
[0041] Purification of rhIDUA
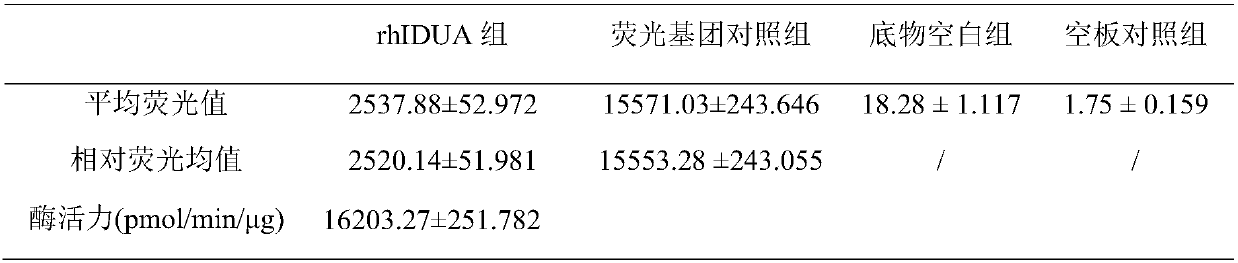
[0042] The supernatant of the above expression cells was purified by ion exchange chromatography, and the specific operations were as follows:
[0043] Anion exchange chromatography: 1mL HiTrap TM The Q HP prepacked column is connected to the AKTA purifier chromatography system. Mobile phase A: 20mM Tris-HCl, pH=8.0; mobile phase B: 20mM Tris-HCl, containing 1M sodium chloride, pH=8.0; flow rate 1.5mL / min, detection wavelength 254nm and 280nm. First equilibrate the chromatography column with mobile phase A for 5-10CV, and load the collected and treated cell supernatant. The target protein is bound to the medium, and some impurities penetrate. After loading the sample, use mobile phase A to wash the chromatography column for 3-5CV, and finally use mobile phase B to carry out gradient elution (0-50% B, 20min) for the target protein, collect the eluted samples and replace the buffer to 20mM citric acid (pH=6.0). The column was regenerated wi...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap