Catalyst for isomerization of paraffins and production method thereof
A catalyst and isomerization technology, which is applied in the field of oil refining industry, can solve the problems of insufficient catalyst strength, etc., and achieve the effects of increasing anti-poison, increasing selectivity, and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
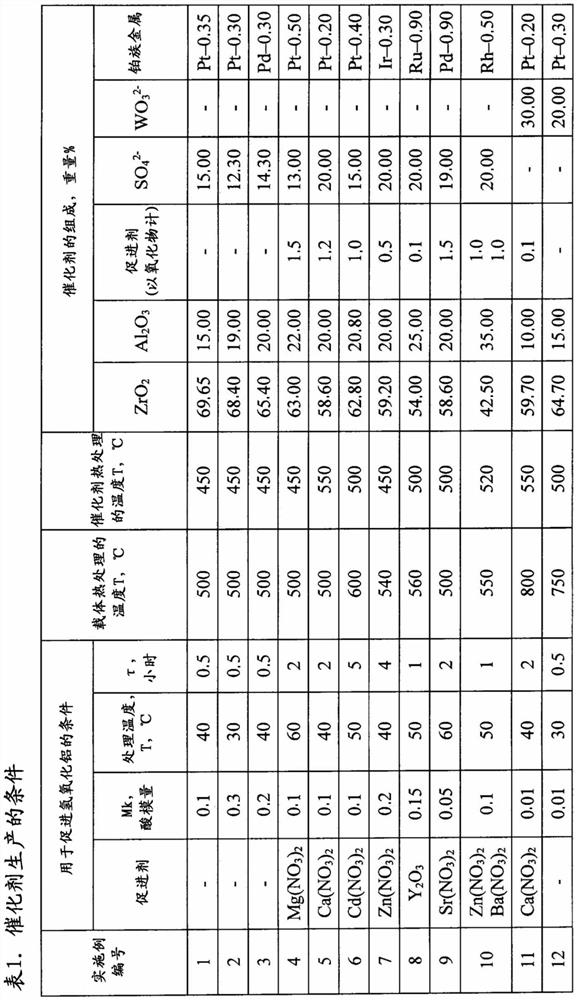
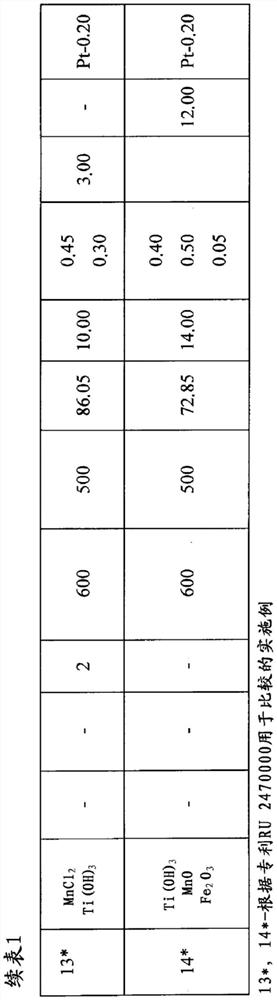
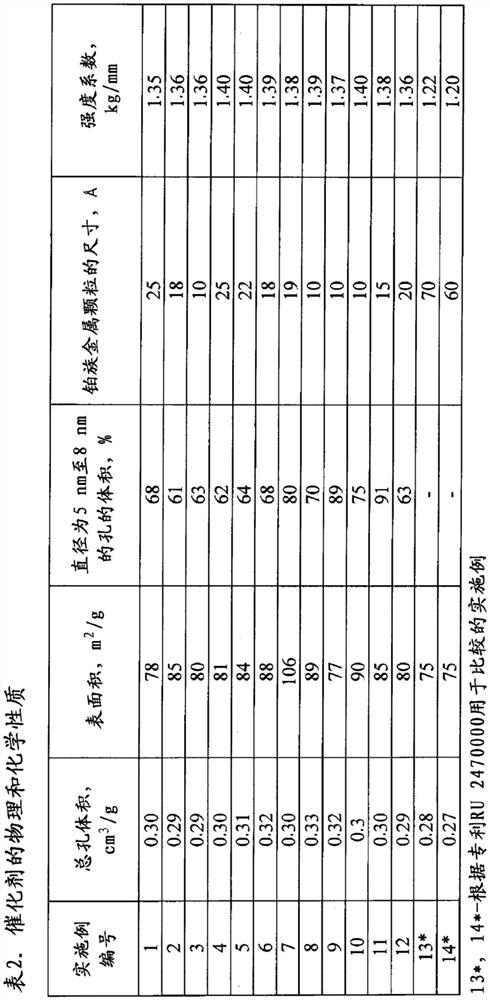
[0117] Add 0.1 mol of HNO to 200g of aluminum hydroxide powder Catapal A with pseudo-boehmite structure 3 / 1mol Al 2 o 3 of nitric acid. The aluminum hydroxide was stirred vigorously, the temperature was raised to 40°C and maintained at this temperature for up to 2 hours.
[0118] The amount of ready-made sulfated zirconium hydroxide required to obtain the desired catalyst composition was added to the acid-treated aluminum hydroxide, mixed vigorously, and the sulfuric acid was added to the catalyst with the predetermined sulfate ion content.
[0119] The resulting mixture was formed by extrusion, dried in a muffle furnace at a temperature of 120°C to 160°C, and heat-treated at 500°C for 2 hours. A chloroplatinic acid solution was added to the obtained carrier according to the initial humidity. The resulting catalyst charge was dried at a temperature of 100°C to 150°C for 3 hours and heat-treated in an air stream of 450°C for 2 hours; the catalyst composition is shown in Ta...
Embodiment 2
[0122] Similar to Example 1, but the difference is that the use contains 0.3 moles of CH 3 COOH / 1 mole Al 2 o 3 of acetic acid as the acid. When impregnating the support, platinum acetate was applied using cyclic impregnation. The composition of the catalyst is shown in Table 1.
[0123] Similar to Example 1, the catalyst was tested during n-butane isomerization, but with the difference that the hydrogen:hydrocarbon molar ratio = 0.2:1.
Embodiment 3
[0125] Similar to Example 1, but the difference is that the acid used is containing 0.2 mol HCOOH / 1 mol A1 2 o 3 Formic acid, a solution of palladium acetate to sodium acetate in a weight ratio of 2.00:0.10 was used during impregnation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com