A kind of aromatic hydrocarbon ruthenium complex and its preparation method and application
A technology of ruthenium complexes and aromatic hydrocarbons, applied in ruthenium organic compounds, chemical instruments and methods, platinum group organic compounds, etc., to achieve good anti-tumor effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] [(η 6 -p-cymene)RuCl] 2 Cl 2 preparation of
[0106] RuCl 3 With 1-methyl-4-isopropyl-1,3-cyclohexadiene, at 50°C, microwave radiation for 30min, suction filtration, to obtain [(η 6 -p-cymene)RuCl] 2 Cl 2 .
Embodiment 2
[0108] [(η 6 Synthesis of -p-cymene)Ru(o-ClPIP)Cl]Cl (No. RAP051):
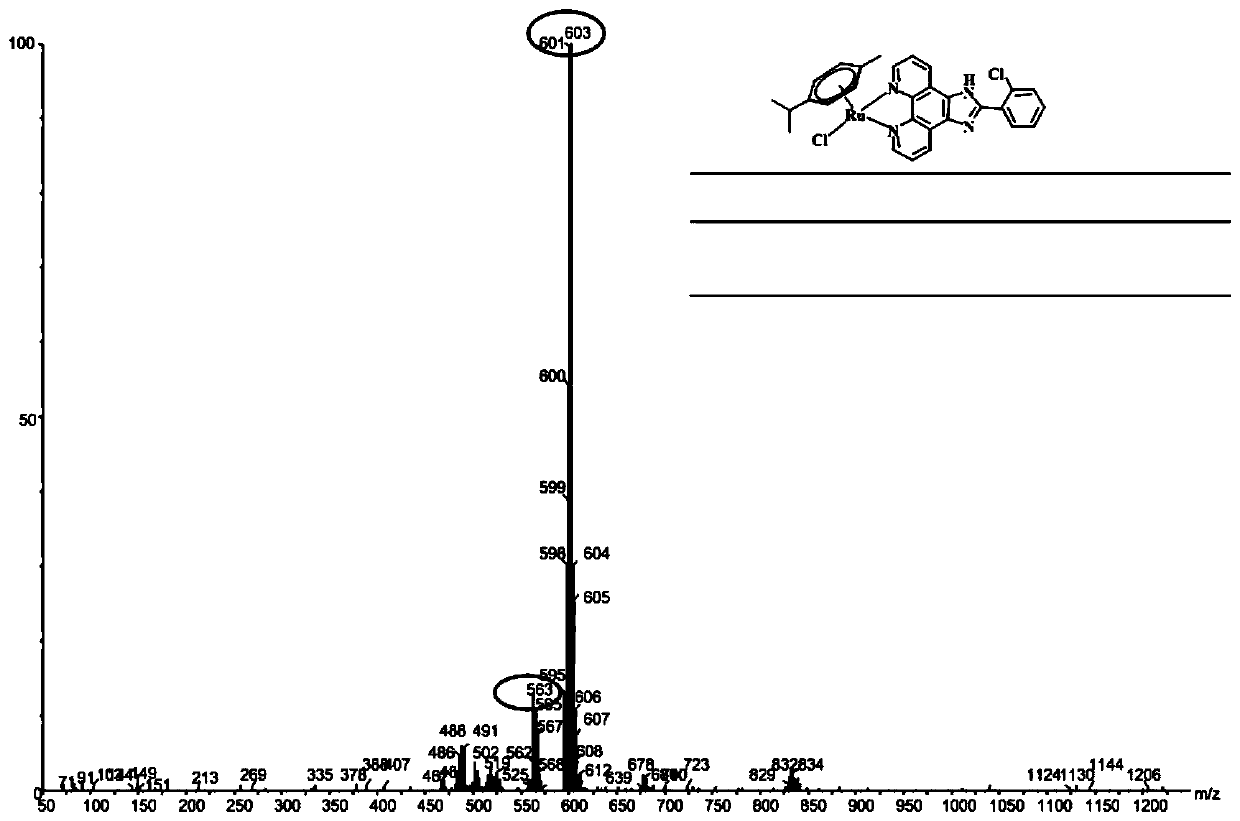
[0109] Get the phellandrene precursor that embodiment 1 obtains [(η 6 -p-cymene)RuCl] 2 Cl 2 (0.1mmol, 61.2mg), ligand o-ClPIP (0.2mmol, 66.1mg), at 60°C, microwave irradiation for 15s, to obtain 124.5mg of bright orange-yellow solid, which is [(η 6 -p-cymene)Ru(o-ClPIP)Cl]Cl(RAP051), yield 97.9%.
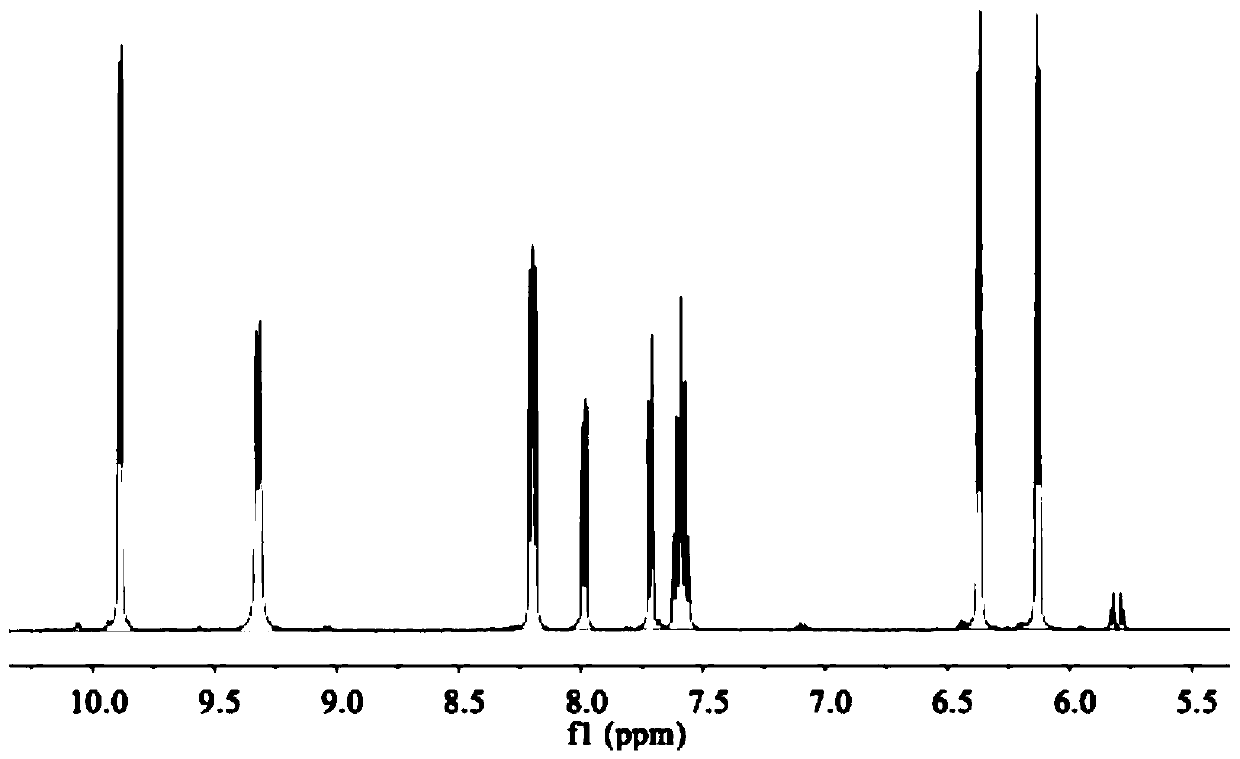
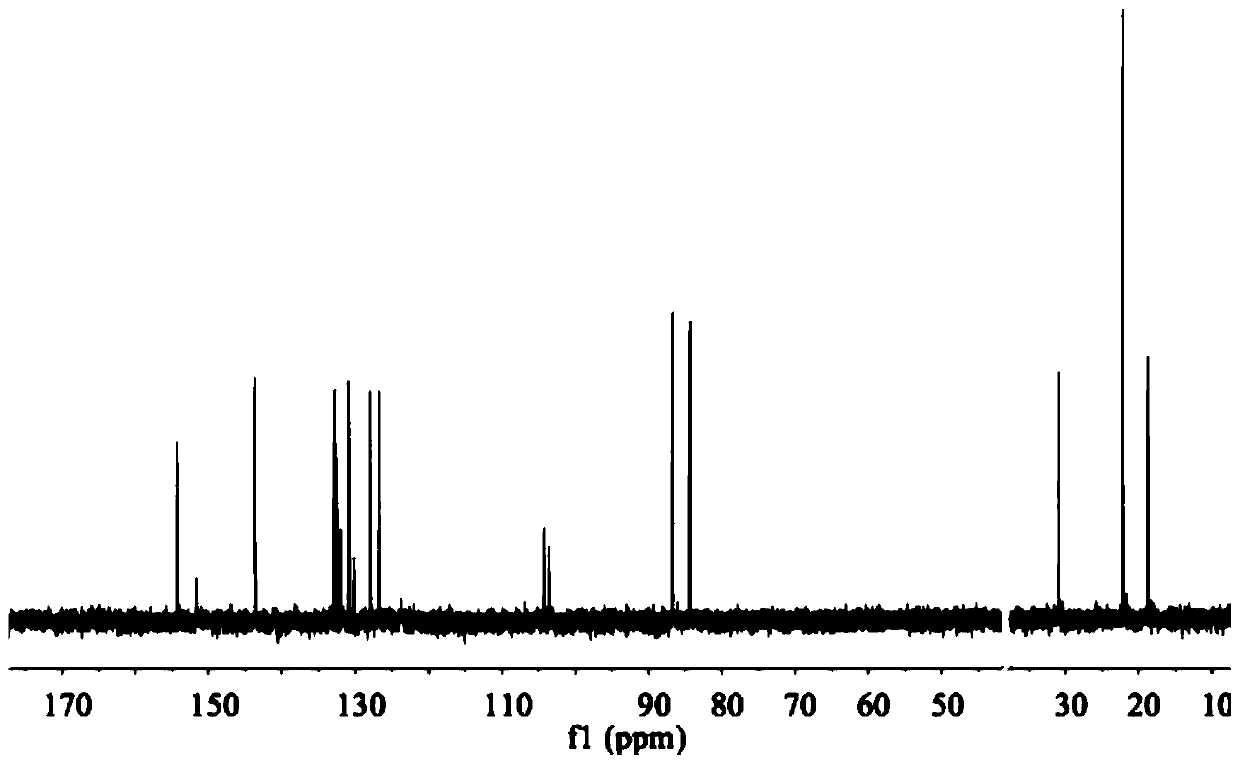
[0110] The ESI-MS spectrum of RAP051 is as follows Figure 1-Figure 3 Shown, ESI-MS (in MeOH, m / z): 636 (Cal.), 601 (Foundfor[M-Cl] + ), 565 (Found for [M-2Cl-H] + ). 1 H NMR (500MHz, DMSO-d 6 ( dd,J=23.2,8.0Hz,1H), 7.63–7.55(m,1H), 6.37(d,J=6.2Hz,1H), 6.13(d,J=6.2Hz,1H), 2.23(d,J =9.5Hz, 1H), 1.47–1.06(m, 1H), 0.91(d, J=6.9Hz, 3H). 13 C NMR (126MHz, DMSO-d 6 )δ154.24(s), 152.11–148.43(m), 143.64(s), 134.16–132.32(m), 131.98(s), 130.89(s), 130.55(d, J=85.9Hz), 127.95(s ), 126.70(s), 104.28(s), 103.57(s), 86.73(s), 84.38(s), 22.13(s), 18.77(s).
Embodiment 3
[0112] [(η 6 Synthesis of -p-cymene)Ru(PIP)Cl]Cl (No. RAP09):
[0113] Get the phellandrene precursor that embodiment 1 obtains [(η 6 -p-cymene)RuCl] 2 Cl 2 (0.1mmol, 61.2mg), ligand PIP (0.2mmol, 59.2mg), at 60°C, microwave irradiation for 30min, an orange-yellow solid was obtained, 113mg, yield 91.0%. ESI-MS (in MeOH, m / z) :592(Cal.),567(Found for[M-Cl] + ). 1 H NMR (500MHz, DMSO-d 6)δ9.85(d, J=5.2Hz, 1H), 9.45(s, 1H), 8.48(d, J=7.9Hz, 1H), 8.19(dd, J=8.1, 5.3Hz, 1H), 7.59( dd,J=20.7,13.2Hz,1H),7.55(dd,J=20.2,13.0Hz,1H),6.35(d,J=6.2Hz,1H),6.12(d,J=6.2Hz,1H), 2.82–2.52(m,1H),2.23(d,J=19.7Hz,2H),1.30–1.06(m,1H),0.89(t,J=15.6Hz,3H). 13 C NMR (126MHz, DMSO-d 6 )δ153.53(s), 143.02(s), 132.60(s), 130.03(s), 128.99(s), 126.74(s), 126.05(s), 103.79(s), 102.91(s), 86.19( s), 83.91(s), 73.23–27.78(m), 21.53(d, J=16.7Hz), 18.24(s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com