Non-solvated crystals as well as preparation method and application thereof
A non-solvated, crystal technology, applied in the direction of organic chemical methods, non-central analgesics, anti-inflammatory agents, etc., can solve the problem of not preparing non-solvated crystals, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of non-solvated crystal A of N-(2-aminophenyl)-6-(7-methoxyquinoline-4-oxygen)-1-naphthylcarboxamide
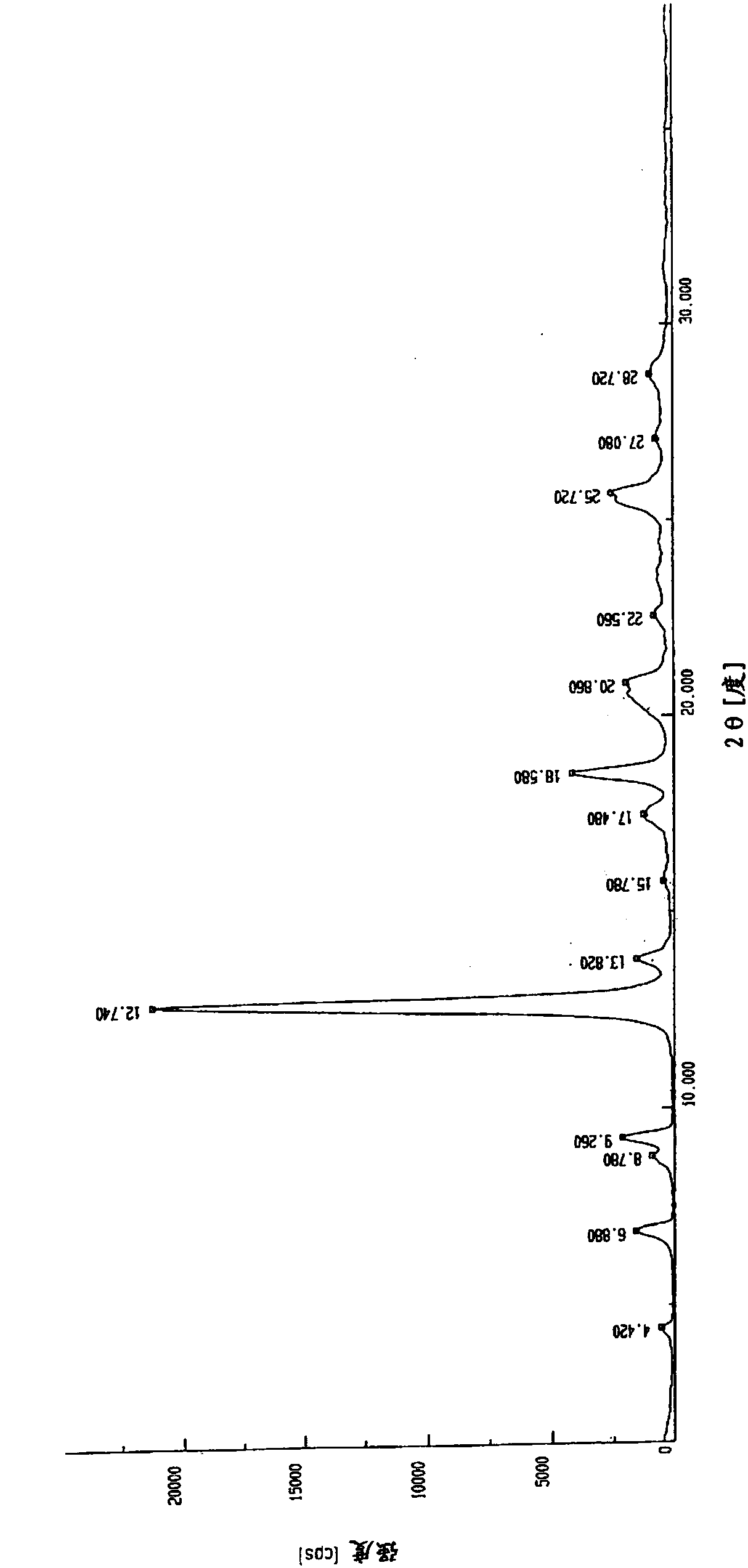
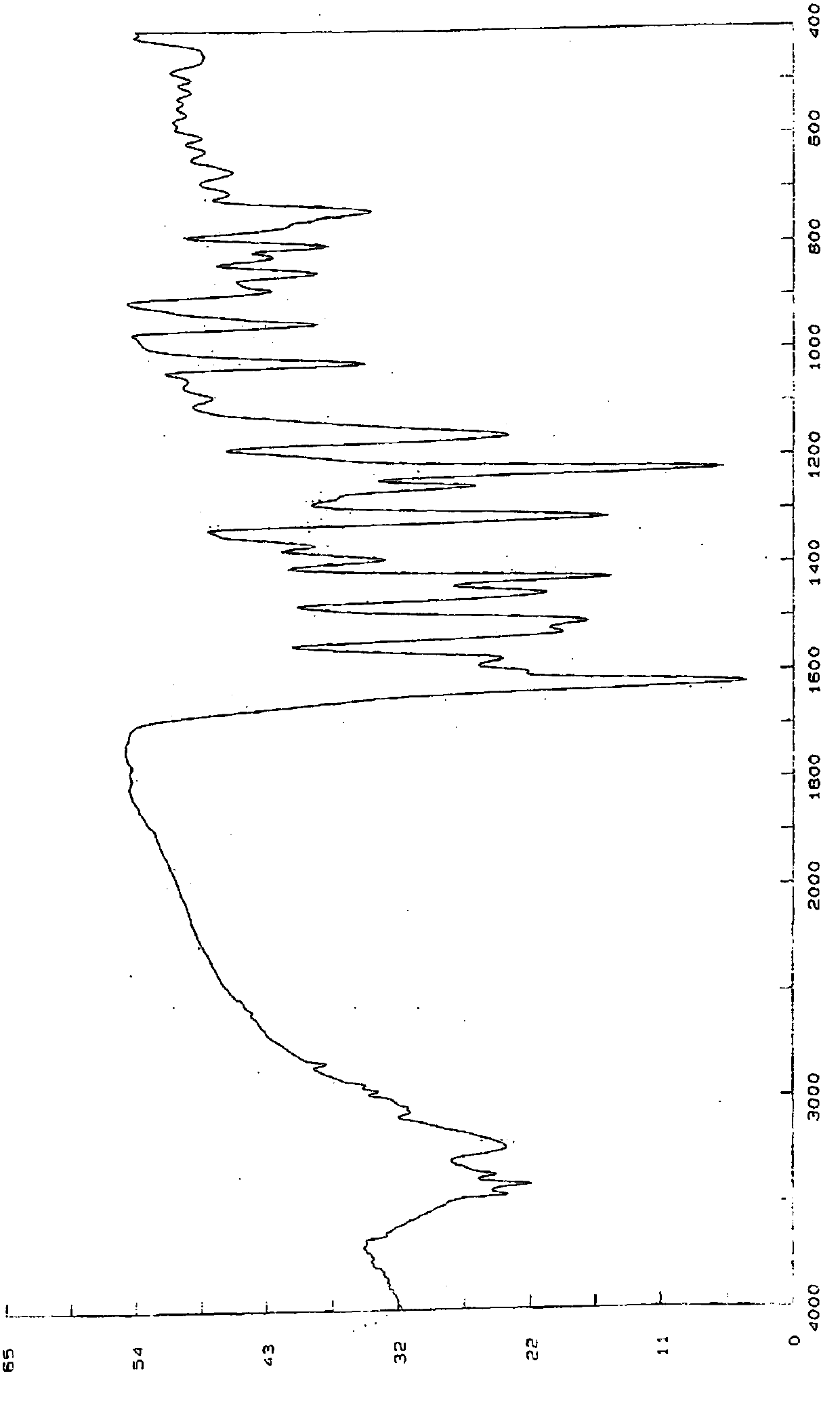
[0049] Put 5.0g of N-(2-aminophenyl)-6-(7-methoxyquinoline-4-oxo)-1-naphthylcarboxamide in a 2000mL three-necked flask, add 750mL of methanol, and place in a 65°C oil bath Heat and stir until dissolved. The resulting solution was cooled and crystallized in an ice-water bath at 0°C for 4 hours, the solid was collected by filtration, and dried in vacuum at 80°C for 12 hours to obtain non-solvated crystal A. Its X-ray powder diffraction pattern is as figure 2 As shown, there are characteristic peaks at reflection angles 2θ of about 4.42°, 6.88°, 8.78°, 9.26°, 12.74°, 13.82°, 15.78°, 18.58°, 20.86°, 22.56°, 25.72°, 27.08° and 28.72° ; Its infrared spectrum is as image 3 Shown at approximately 3452, 3404, 3357, 3230, 3064, 1622, 1576, 1525, 1506, 1452, 1423, 1388, 1363, 1311, 1253, 1224, 1161, 1088 and 1024cm -1 There is a characteristic absorpt...
Embodiment 2
[0050] Example 2: Preparation of non-solvated crystal B of N-(2-aminophenyl)-6-(7-methoxyquinoline-4-oxygen)-1-naphthylcarboxamide
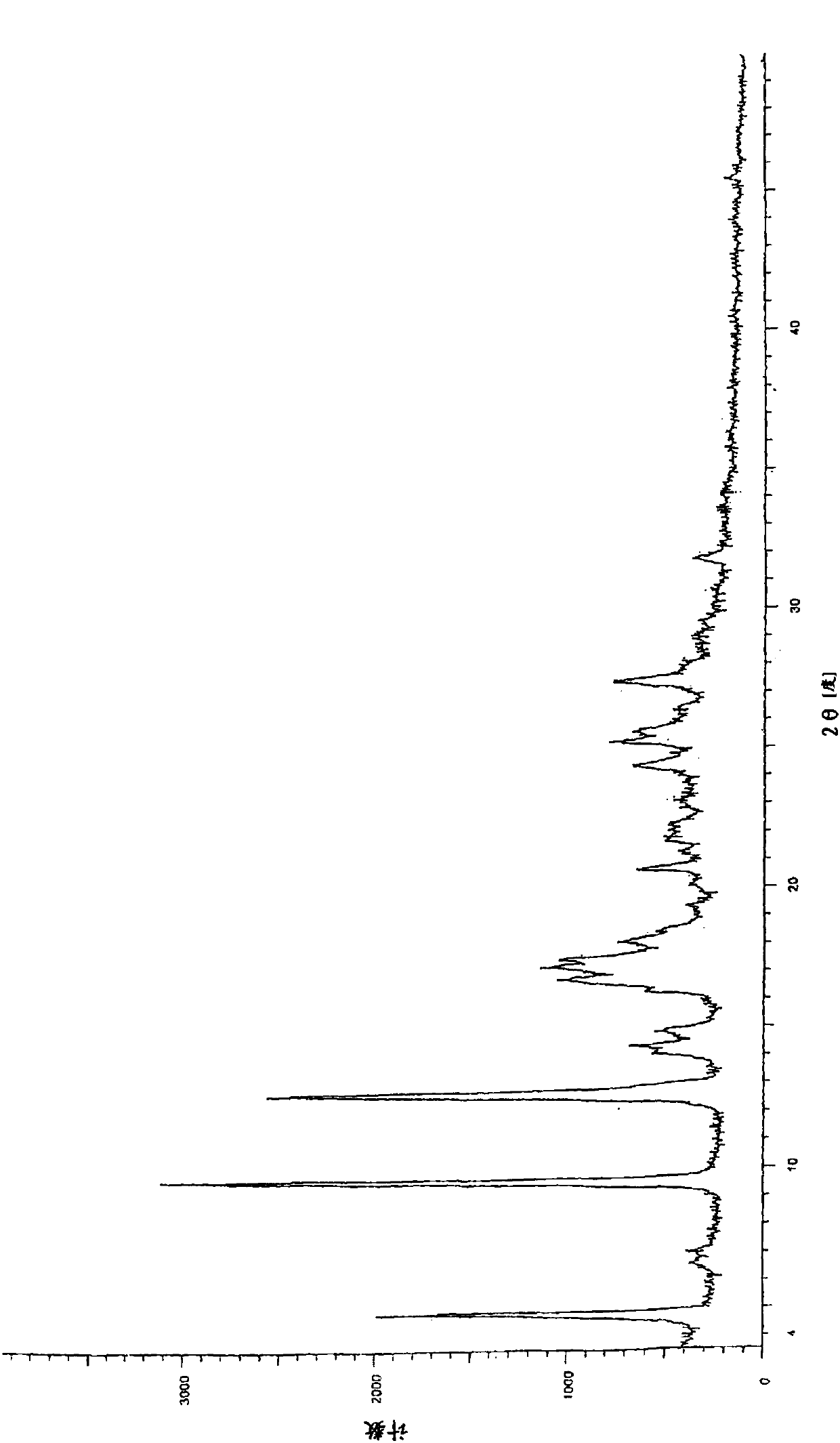
[0051] Put 5.0g of N-(2-aminophenyl)-6-(7-methoxyquinoline-4-oxo)-1-naphthylcarboxamide in a 2000mL three-neck flask, add 1000mL of acetonitrile, and place in an oil bath at 80°C Heat and stir until dissolved. The resulting solution was cooled and crystallized in an ice-water bath at 0°C for 4 hours, the solid was collected by filtration, and dried in vacuum at 80°C for 12 hours to obtain non-solvated crystal B. The X-ray powder diffraction pattern of the crystal is shown in Figure 5 shown. Its X-ray powder diffraction pattern has characteristic peaks at about 4.88°, 9.68°, 12.74°, 14.52°, 17.72°, 19.82°, 21.86°, 24.30°, 25.26° at reflection angle 2θ; its infrared spectrum is as follows: Image 6 As shown, at about 3423, 3352, 3238, 3030, 1624, 1597, 1531, 1502, 1452, 1423, 1388, 1365, 1308, 1255, 1226, 1159, 1086, 1022cm -1 There is a chara...
Embodiment 3
[0052] Example 3: Preparation of non-solvated crystal C of N-(2-aminophenyl)-6-(7-methoxyquinoline-4-oxygen)-1-naphthylcarboxamide
[0053] Put 1.0g N-(2-aminophenyl)-6-(7-methoxyquinoline-4-oxo)-1-naphthylcarboxamide in a 50mL three-necked flask, add 5mL dimethyl sulfoxide, and stir at room temperature until dissolved. The obtained solution was dripped into 50 mL of water under stirring, stood still for 4 hours, filtered, washed with water, collected solid, and dried in vacuum at 80° C. for 24 hours to obtain non-solvated crystal C. The X-ray powder diffraction pattern of the crystal is shown in Figure 8 As shown, its X-ray powder diffraction pattern is about 4.84°, 9.68°, 12.92°, 14.60°, 16.46°, 17.20°, 17.44°, 17.88°, 19.20°, 20.54°, 21.06°, 22.00° at reflection angle 2θ , 25.28°, and 27.66° have characteristic peaks; its infrared spectrum is as Figure 9 As shown, there are characteristic absorptions at about 3452, 3369, 3217, 3016, 2962, 1793, 1728, 1626, 1595, 1574, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com