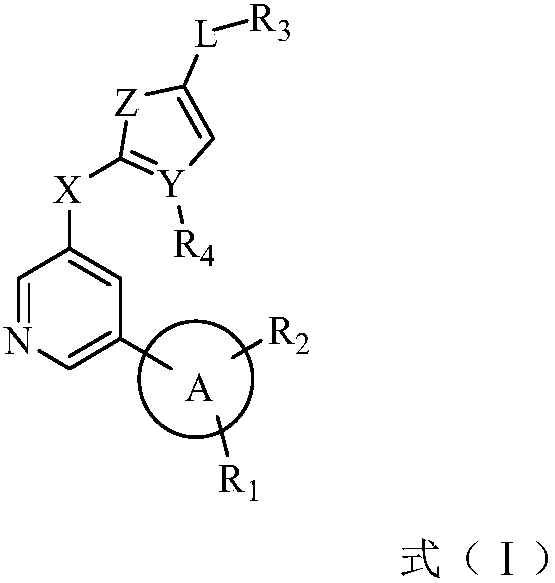
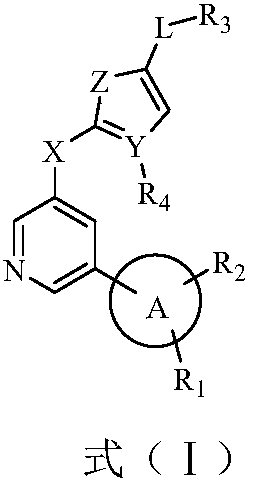
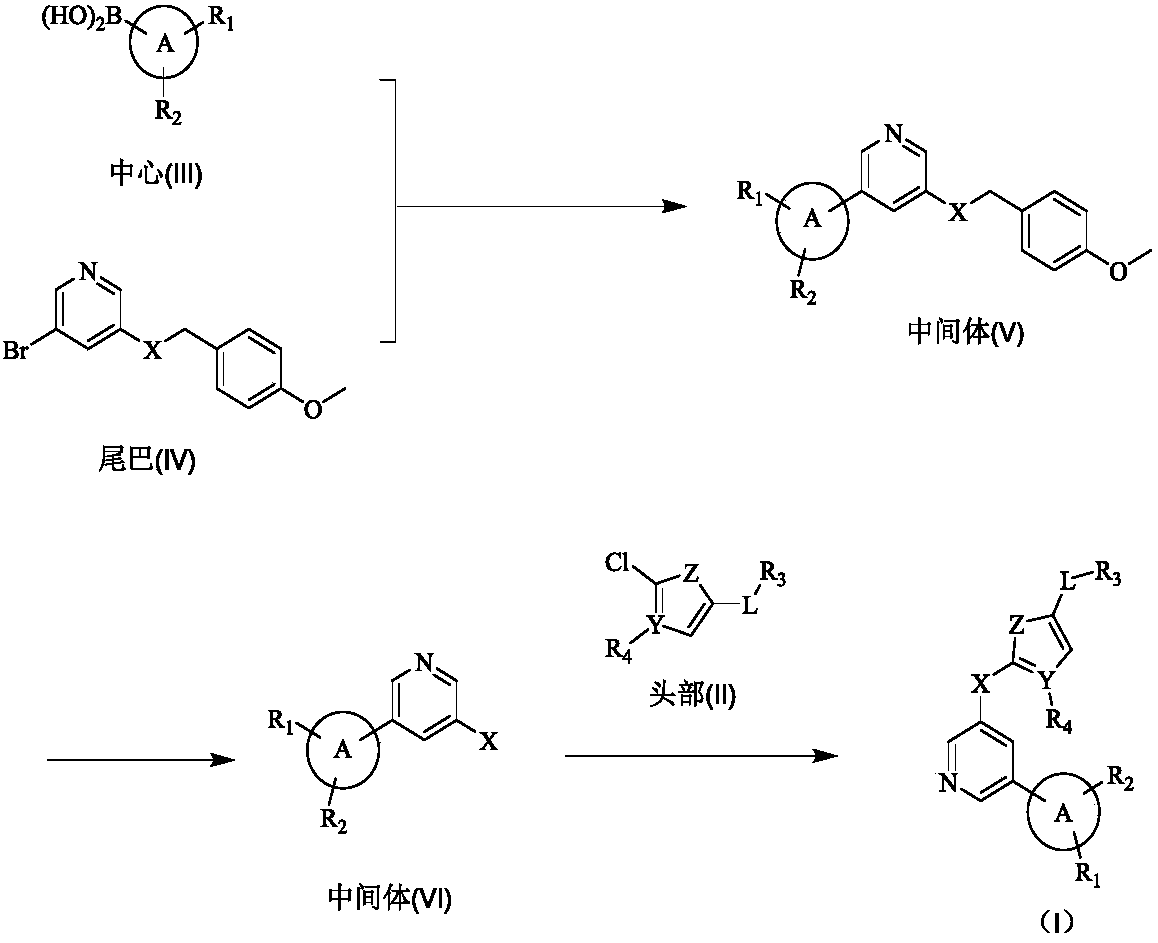
Biaryl pyridine deubiquitinating enzyme inhibitor as well as preparation method and application thereof
A pyridine and aryl technology, applied in the application field of preparing anti-tumor drugs, can solve the problems of low efficiency, unsatisfactory micromolar level specificity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Example 1 3-bromo-5-(4-methoxybenzylthio)pyridine
[0104]
[0105] 4-Methoxybenzylthiol (32.55g, 211.07mmol) was dissolved in 500mL dimethylformamide, NaH (9.29g, 232.18mmol) was added, and reacted at 20°C for 30min, then, 3,5-dibromopyridine ( 1,50.00g, 211.07mmol), react at 20°C for 16 hours, add 100mL of water to terminate the reaction, extract with ethyl acetate (100mL x 3), wash the organic layer with water (200mL x 3), dry over anhydrous sodium sulfate, and recover under reduced pressure The solvent was separated by column chromatography to obtain 48.0 g of white solid (compound 2), with a yield of 73%. 1 HNMR (400MHz, CDCl 3 ): δ=8.38(d, J=2.1Hz, 1H), 8.31(d, J=1.9Hz, 1H), 7.60(t, J=2.0Hz, 1H), 7.13-7.08(m, 2H, Ar- H),6.79-6.73(m,2H,Ar-H),4.00(s,2H,CH 2 ),3.71(s,3H,CH 3 ).ESI-MS: m / z=311[M+H] + .
Embodiment 2
[0106] Example 2 3-(2-isopropylphenyl)-5-(4-methoxybenzylthio)pyridine
[0107]
[0108] Compound 2 (15.00g, 48.35mmol), 2-isopropylphenylboronic acid (8.72g, 53.19mmol), Pd(dppf)Cl 2 (3.54g, 4.84mmol) and sodium carbonate (10.25g, 96.71mmol) were added to dioxane / water (10 / 1) 150mL, stirred and reacted at 100°C for 16 hours, after the reaction was completed, the solvent was recovered under reduced pressure, and 20mL of water was added , extracted with dichloromethane (50mL x 3), dried over anhydrous sodium sulfate, recovered the solvent under reduced pressure, and obtained 13.6g of yellow oil (compound 3) by column chromatography, with a yield of 78%. 1 H NMR (400MHz, CDCl 3 ): δ=8.52(d, J=2.2Hz, 1H, pyridine-H), 8.36(d, J=1.9Hz, 1H, pyridine-H), 7.48(t, J=2.1Hz, 1H, pyridine-H ),7.42-7.35(m,2H,Ar-H),7.25-7.16(m,2H,Ar-H),7.11-7.02(m,1H,Ar-H),6.81(d,J=8.6Hz, 2H,Ar-H),4.10(s,2H,CH 2 ),3.78(s,3H,CH 3 ),2.86(q,J=13.7,6.8Hz,1H,CH),1.15(t,J=10.8Hz,6H,CH 3 ).ESI-MS: m / z=359...
Embodiment 3
[0109] Example 3 3-benzyl-5-(4-methoxybenzylthio)pyridine
[0110]
[0111] Synthesis of Compound 4: Using Compound 2 and 2-ethylphenylboronic acid as raw materials, the synthesis and post-treatment were the same as in Example 2. 11.7 g of a yellow oil (compound 4) was obtained, yield 72%. 1 H NMR (400MHz, CDCl 3): δ=8.52(d, J=2.1Hz, 1H, pyridine-H), 8.38(d, J=1.8Hz, 1H, pyridine-H), 7.50(t, J=2.0Hz, 1H, pyridine-H ),7.39–7.15(m,5H,Ar-H),7.10(d,J=7.7Hz,1H,Ar-H),6.81(d,J=8.6Hz,2H,Ar-H),4.11(d ,J=7.4Hz,2H,CH2),3.78(s,3H,CH3),2.50(q,J=7.5Hz,2H,CH2),1.07(t,J=7.5Hz,3H,CH3).ESI- MS:m / z=336.5[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com