Storage stable compositions and methods for the treatment of refractive errors of the eye
A composition and ophthalmic technology, applied in the fields of aceclidinium composition, muscarinic agonist, and composition for treating presbyopia, which can solve problems such as improper recombination and improper treatment of presbyopia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0164] In another embodiment, the ophthalmic composition comprises:
[0165] Aceclidinium at a concentration of about 1.75% w / v;
[0166] Tropicamide at a concentration of about 0.042% w / v;
[0167] Macrogol 40 stearate at a concentration of about 4.5% w / v;
[0168] Mannitol at a concentration of about 2.5% w / v;
[0169] Acetate buffer at a concentration of about 3.0 mM; and
[0170] BAK at a concentration of about 0.02% w / v,
[0171] wherein said composition has a pH of about 4.75.
[0172] In another embodiment, the ophthalmic composition comprises:
[0173] Aceclidinium at a concentration of about 1.55% w / v;
[0174] Tropicamide at a concentration of about 0.042% w / v;
[0175] Macrogol 40 stearate at a concentration of about 5.5% w / v;
[0176] Citric acid monohydrate at a concentration of about 0.1% w / v;
[0177] Mannitol at a concentration of about 4.0% w / v;
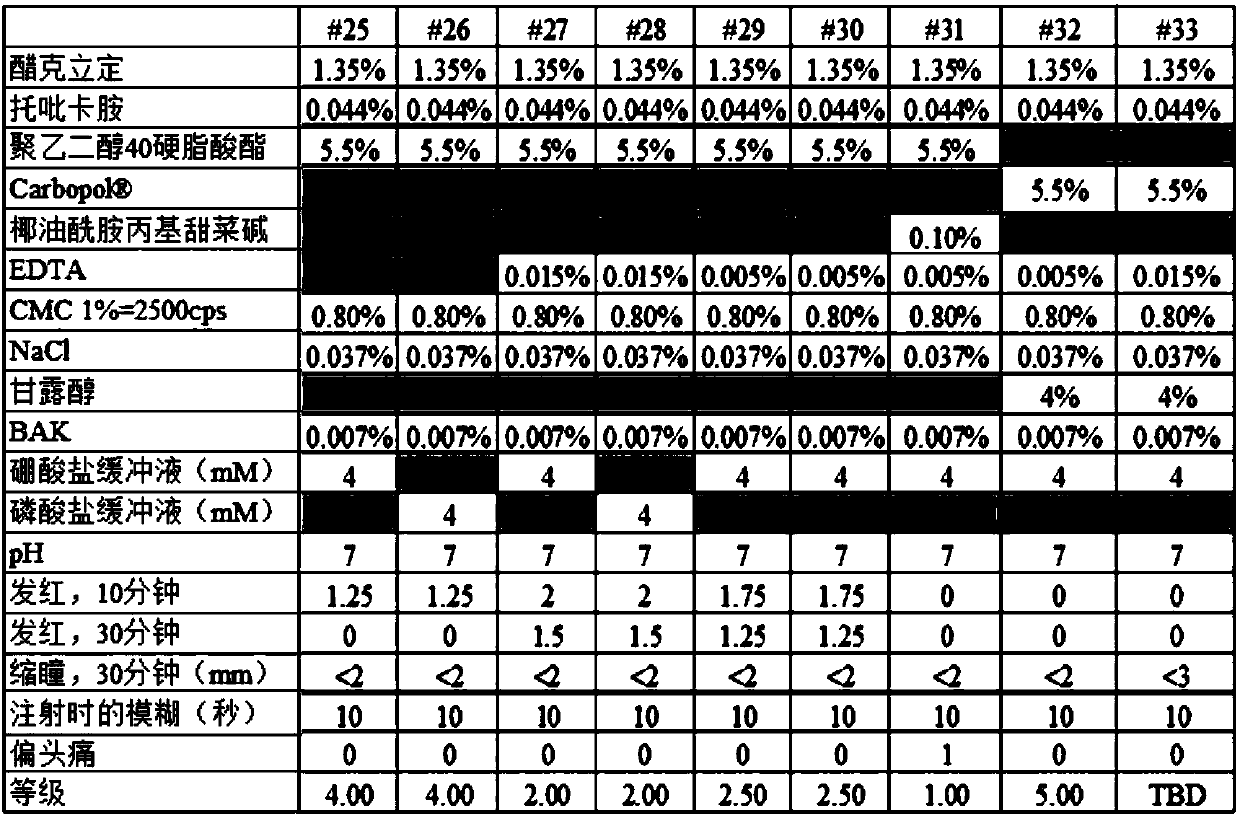
[0178] Concentration of 0.09% w / v (Carbomer) 940; and acetate buffer at a concentration of about 3.0 mM;...
Embodiment 1
[0227] The influence of embodiment 1-acecidine on the visual acuity of 47 to 67 years old subjects
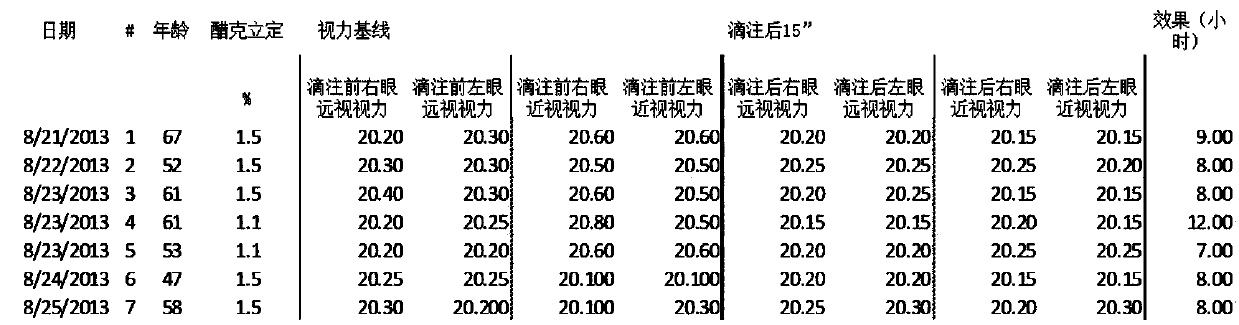
[0228] Table 1 shows the effect on the near-point focusing ability of presbyopic subjects before and after ocular administration of an ophthalmic composition comprising acecidine. Each composition included aceclidine at the concentrations indicated and 5.5% w / v HPβCD, 0.75% w / v CMC, 0.25% w / v NaCl and 0.01% w / v BAK. Additional compositions administered to Subjects 4 and 5 included 0.125% w / v tropicamide. Since aceclidinine is an enantiomer, its clinical effect may vary with different ratios. For the present study, the best stereoisomer ratio obtained by polarimetric measurements was approximately 50:50.
[0229] Table 1. Effect of acecidine on vision in patients with presbyopia
[0230]
[0231] As can be seen from Table 1, all subjects' left and right eyes (target distance 15 feet from the eye) were below ideal nearsightedness (20.20), and most subjects' farsightedness w...
Embodiment 2
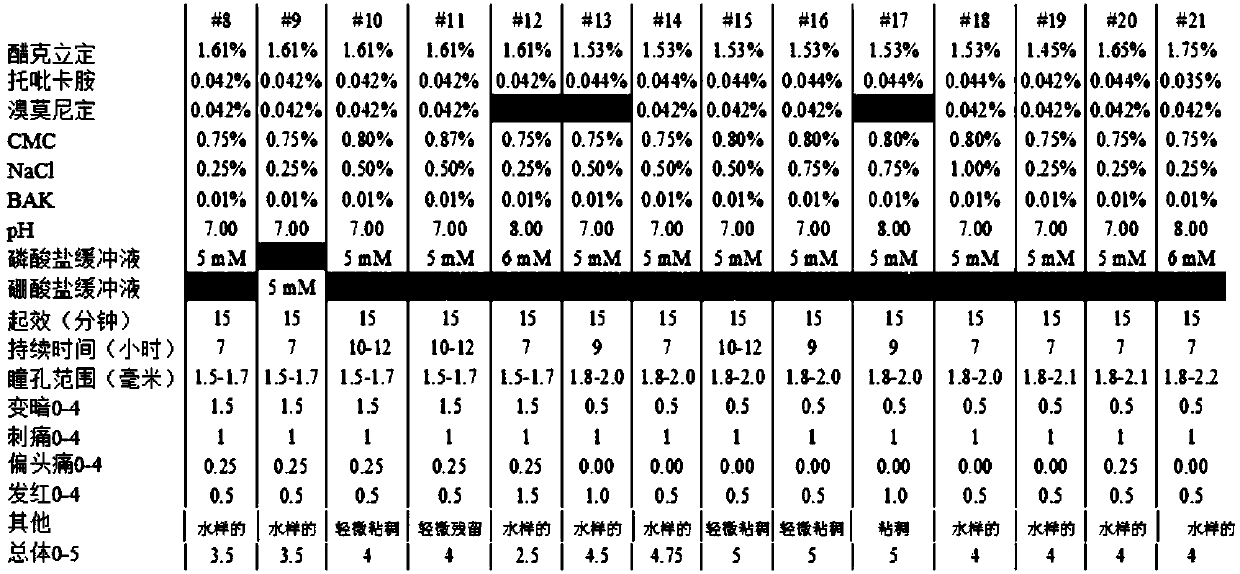
[0232] Example 2 - Concentration Effects of Aceclidine and Tropicamide Concentrations
[0233] Table 2: Concentration Effect of Acecidine and Tropicamide Concentrations
[0234]
#1
#2
#3
#4
#5(OD)
#5(OS)
#6
#7
0.03%
0.03%
0.03%
0.03%
0.03%
0.03%
0.03
Poloxamer 407
5.5%
HPBCD
5.5%
5.5%
5.5%
5.5%
5.5%
5.5%
5.5%
Clinidine
1.5%
1.5%
0.75%
1.1%
1.1%
1.1%
1.1%
1.1%
Tropicamide
0.014%
0.021%
0.028%
0.042%
0.062%
NaCl
0.25%
0.25%
0.25%
0.25%
0.25%
0.25%
0.25%
0.25%
CMC
0.75%
0.75%
0.75%
0.75%
0.75%
0.75%
0.75%
0.75%
BAK
0.1%
0.1%
0.1%
0.1%
0.1%
0.1%
0.1%
0.1%
Redness (15 minutes)
3+
1
0.5
0.5
0
0
0
0
Redness (30 minutes)
1.5
0.5
0.25
0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com