Treatment of leber's hereditary optic neuropathy and dominant optic atrophy with tocotrienol quinones
a technology of tocotrienol quinones and optic nerves, which is applied in the direction of biocide, organic chemistry, drug compositions, etc., can solve the problems of lhon, patients showing no functional improvement, and becoming legally blind, so as to stop the progression of visual activity, improve visual acuity, and stop the progression of color vision loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
LHON Cell Line Assay and Initial Screen for Effective Compounds
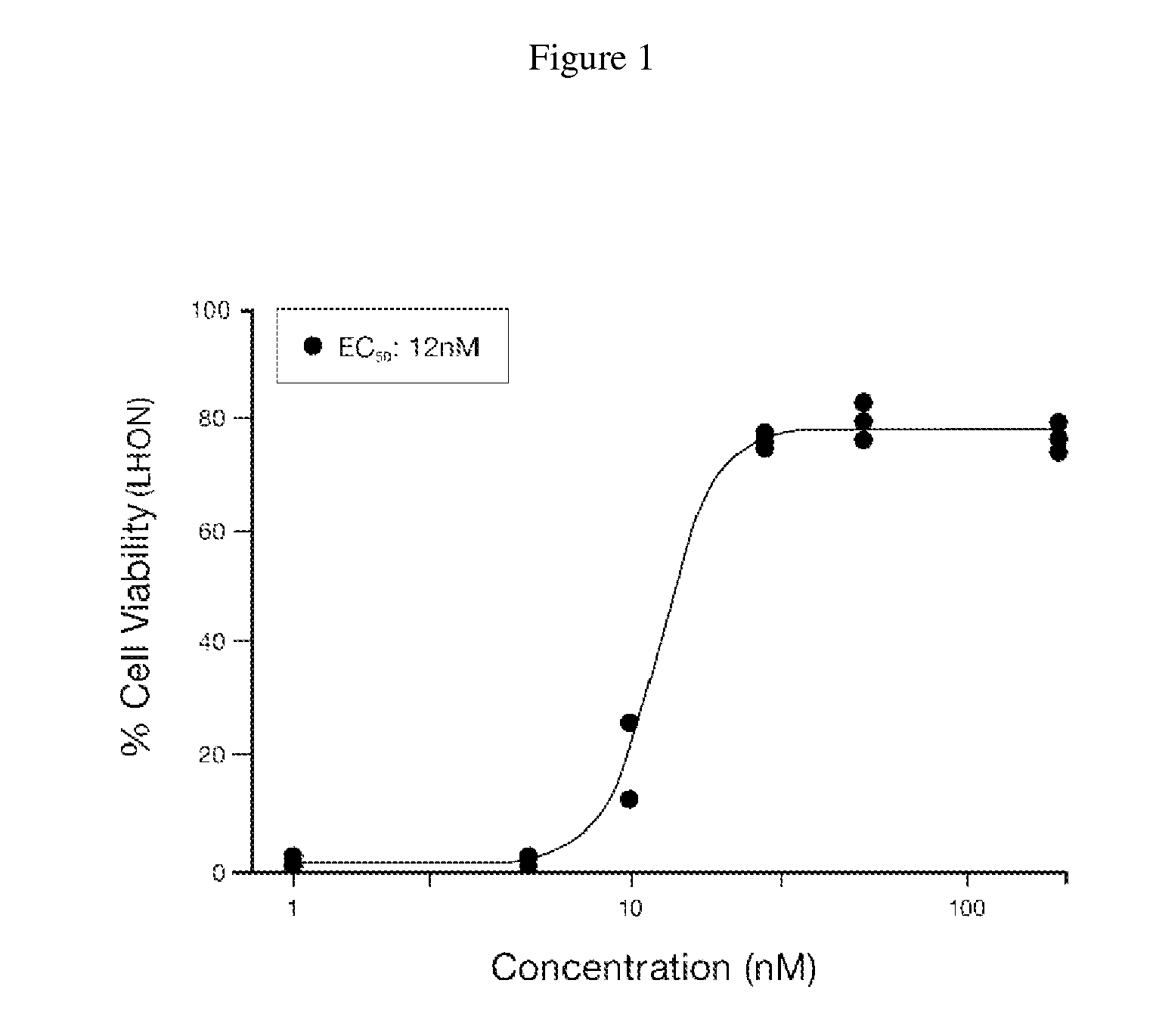
[0129]Alpha-Tocotrienol quinone was tested for its ability to rescue Leber's Hereditary Optic Neuropathy (LHON) fibroblast cells obtained from the Coriell Cell Repositories (Camden, N.J.; repository number GM03858), from stress effected by addition of L-buthionine-(S,R)-sulfoximine (BSO), as described in Jauslin et al., Hum. Mol. Genet. 11(24):3055 (2002), Jauslin et al., FASEB J. 17:1972-4 (2003), and International Patent Application WO 2004 / 003565. EC50 concentrations of test compound and its redox-silent version were determined and compared.
[0130]MEM (a medium enriched in amino acids and vitamins, catalog no. 1-31F24-I) and Medium 199 (M199, catalog no. 1-21F22-I) with Earle's Balanced Salts, without phenol red, were purchased from Bioconcept. Fetal Calf Serum was obtained from PAA Laboratories. Basic fibroblast growth factor and epidermal growth factor were purchased from PeproTech. Penicillin-streptomycin-glutamine ...
example 2
Treatment of a Female LHON Patient (A) Diagnosed with a LHON Mutation
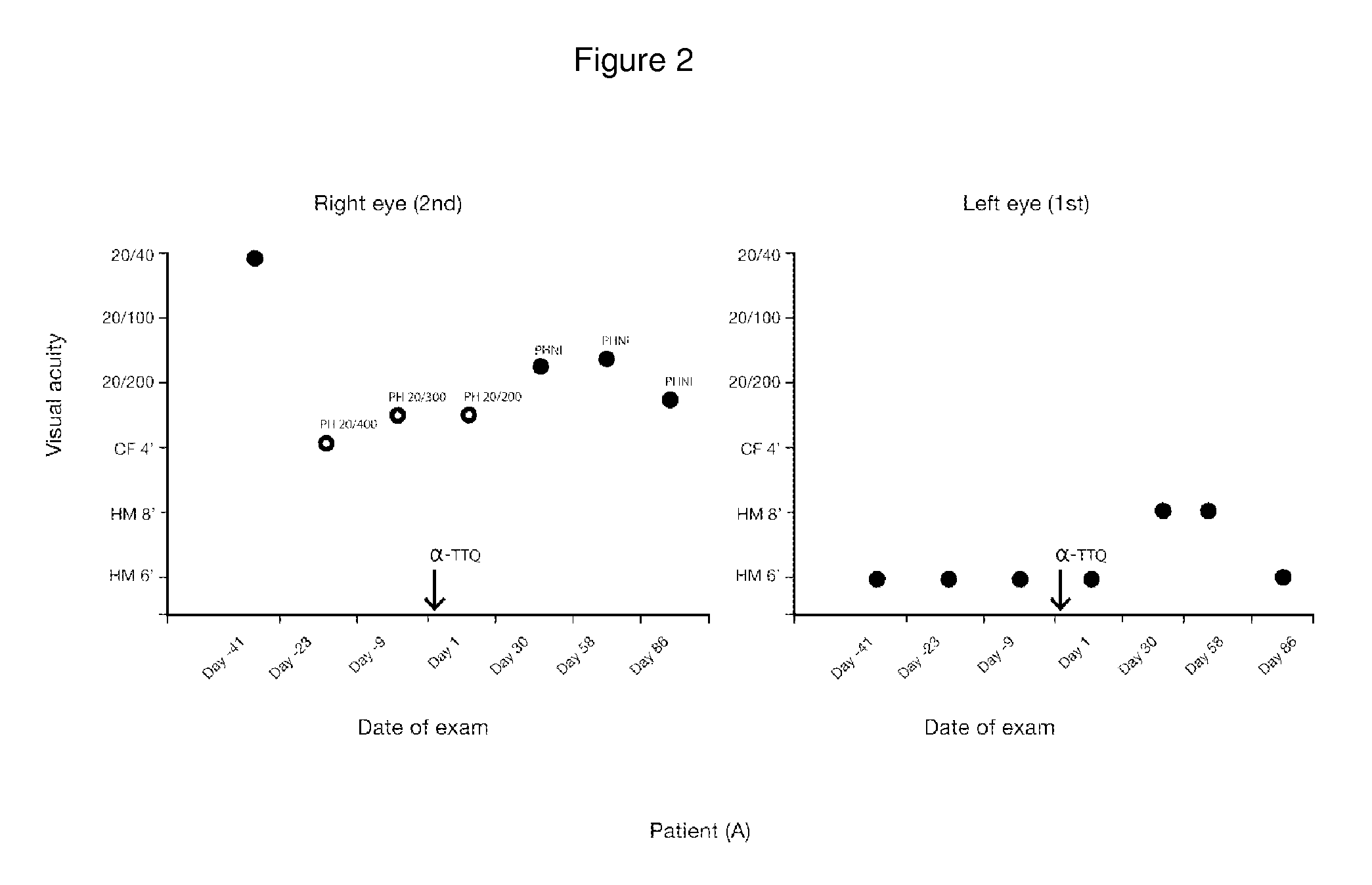
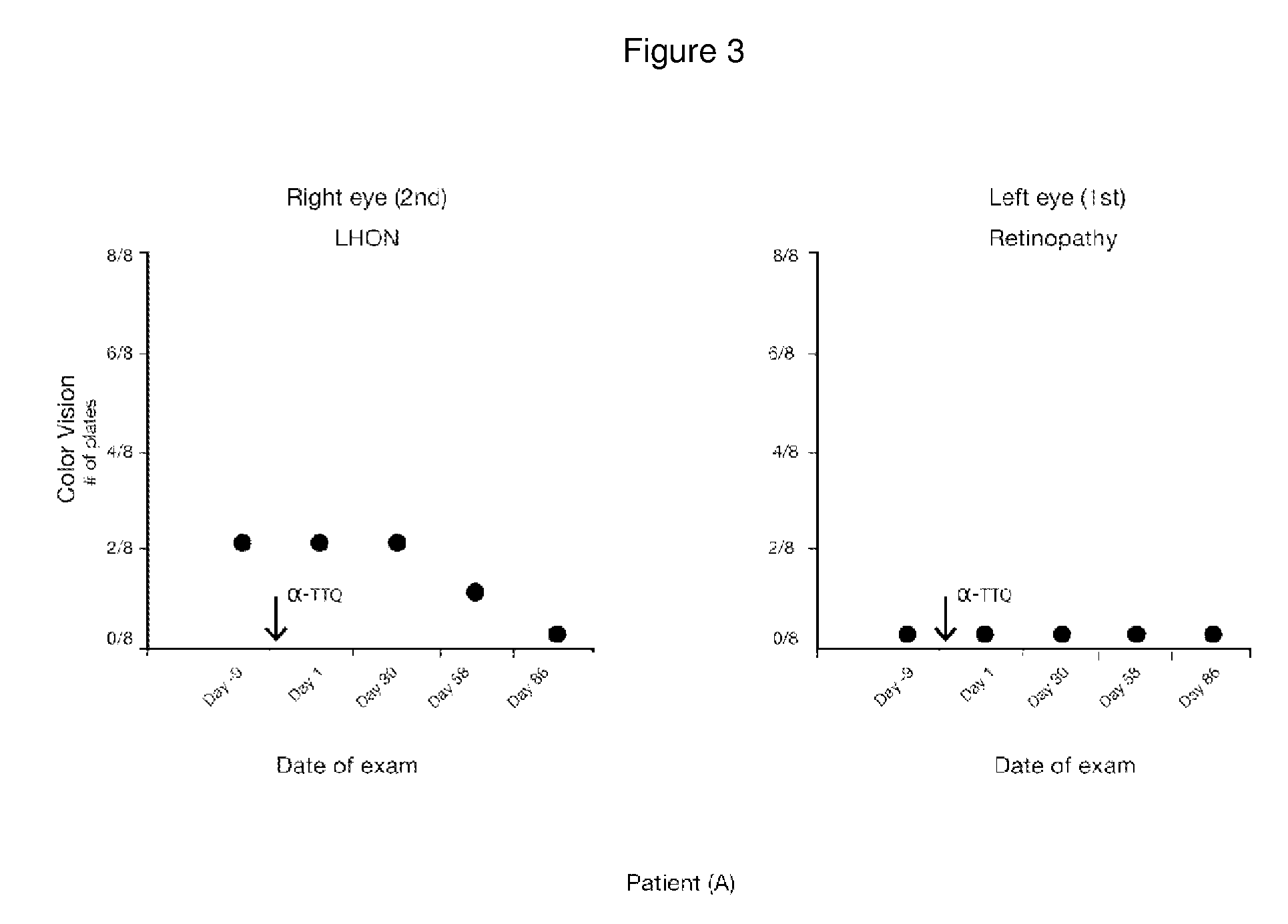
[0139]A fifty two-year-old female patient (A) with a LHON 11778 point mutation was treated with alpha-tocotrienol quinone. Patient (A) also suffered from retinopathy in her left eye when treatment was started.
[0140]Alpha-tocotrienol quinone was administered to the patient orally; the drug was mixed with sesame oil for administration, and the intake was taken with ice-cream. The following dosing of alpha-tocotrienol quinone was used:
On Day 1 the dose was 100 mg TID. It was escalated on Day 8 to 200 mg TID and continued at this dosage.
[0141]While being treated with alpha tocotrienol quinone, the patient's medical team monitored the patient's eyes for any signs of improvement or signs of worsening of the disease.
[0142]The following results, shown in FIGS. 2, 3, 4, and 5, were obtained: (i) absence of loss of visual acuity progression and improvement from 20 / 400 to 20 / 200; (ii) no change in color vision, (iii) improvem...
example 3
Treatment of a Male LHON Patient (B) Diagnosed with a LHON Mutation
[0144]A 23-year-old male patient (B) with a LHON 11778 point mutation was treated with alpha-tocotrienol quinone. At the beginning of treatment the visual acuity was 20 / 400 for the right eye and 20 / 200 for the left eye. As for Patient (A), Patient (B) was treated with a 100 mg TID for seven days and the dose was escalated to 200 mg TID on day 8.
[0145]FIGS. 6, 7, 8 and 9 show the results obtained during the first two months of treatment: (i) absence of loss of visual acuity progression and improvement on right eye from 20 / 400 to 20 / 200 and improvement on left eye from 20 / 200 to 20 / 100; (ii) improvement of color vision in his right eye and maintenance of color vision in his left eye; (iii) improvement in visual fields, and (iv) stable OCT in the left eye.
[0146]Close monitoring of Patient (B) during the study was performed, to detect any adverse events. In addition, the investigator had authority to stop the study if th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com