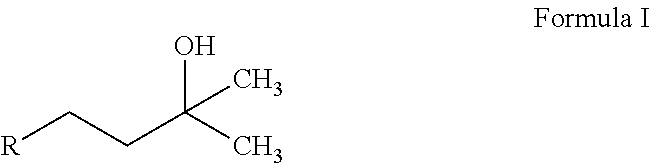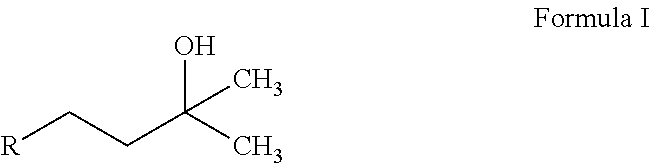Patents
Literature
39 results about "Leber's hereditary optic neuropathy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Leber's hereditary optic neuropathy (LHON) is a mitochondrially inherited (transmitted from mother to offspring) degeneration of retinal ganglion cells (RGCs) and their axons that leads to an acute or subacute loss of central vision; this affects predominantly young adult males. LHON is only transmitted through the mother, as it is primarily due to mutations in the mitochondrial (not nuclear) genome, and only the egg contributes mitochondria to the embryo. LHON is usually due to one of three pathogenic mitochondrial DNA (mtDNA) point mutations. These mutations are at nucleotide positions 11778 G to A, 3460 G to A and 14484 T to C, respectively in the ND4, ND1 and ND6 subunit genes of complex I of the oxidative phosphorylation chain in mitochondria. Men cannot pass on the disease to their offspring.
Treatment of mitochondrial diseases
InactiveUS20050065099A1Limit prevent damageBiocideSenses disorderHuntingtons choreaCerebellar ataxia
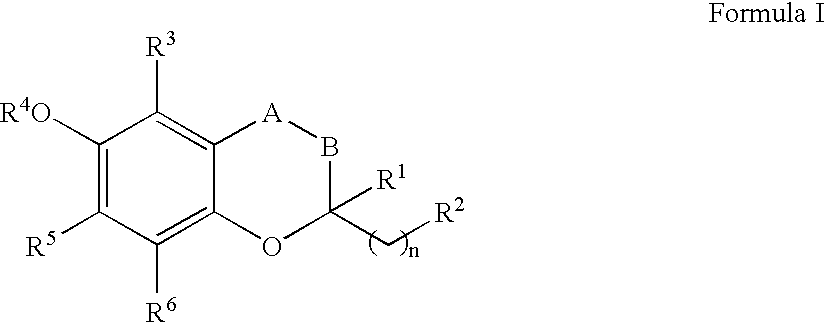
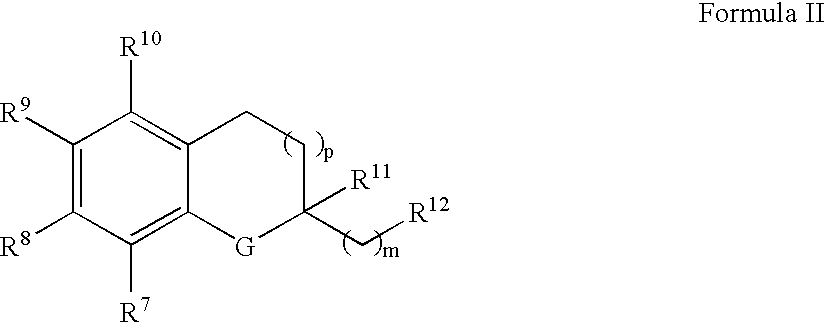
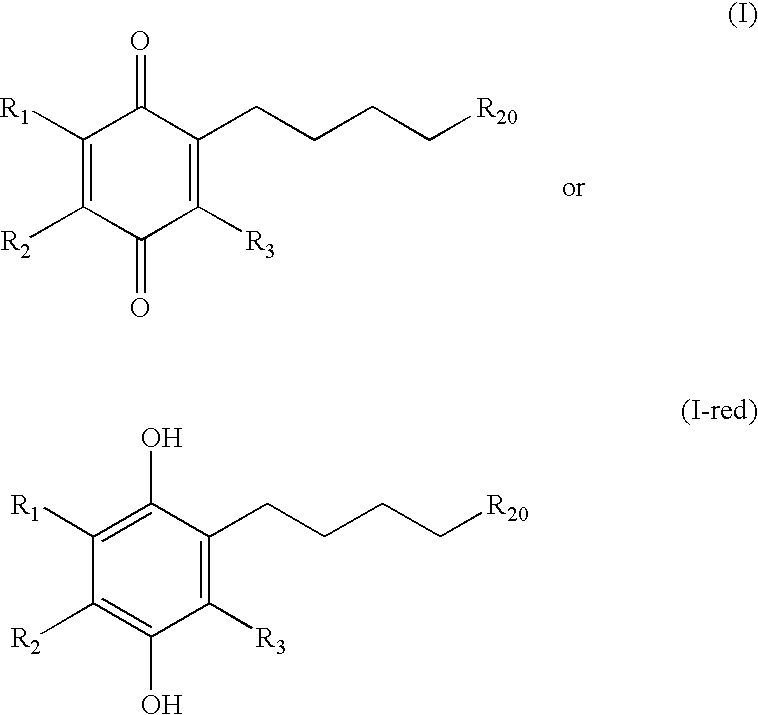
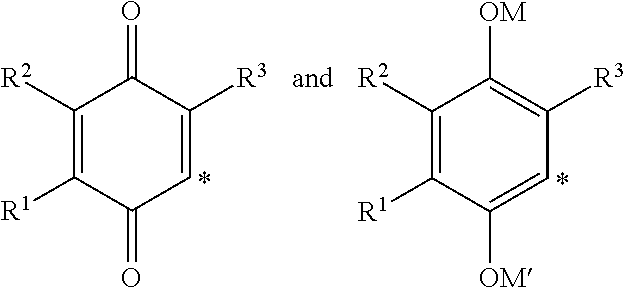
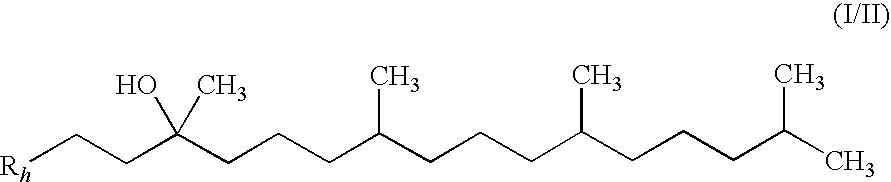
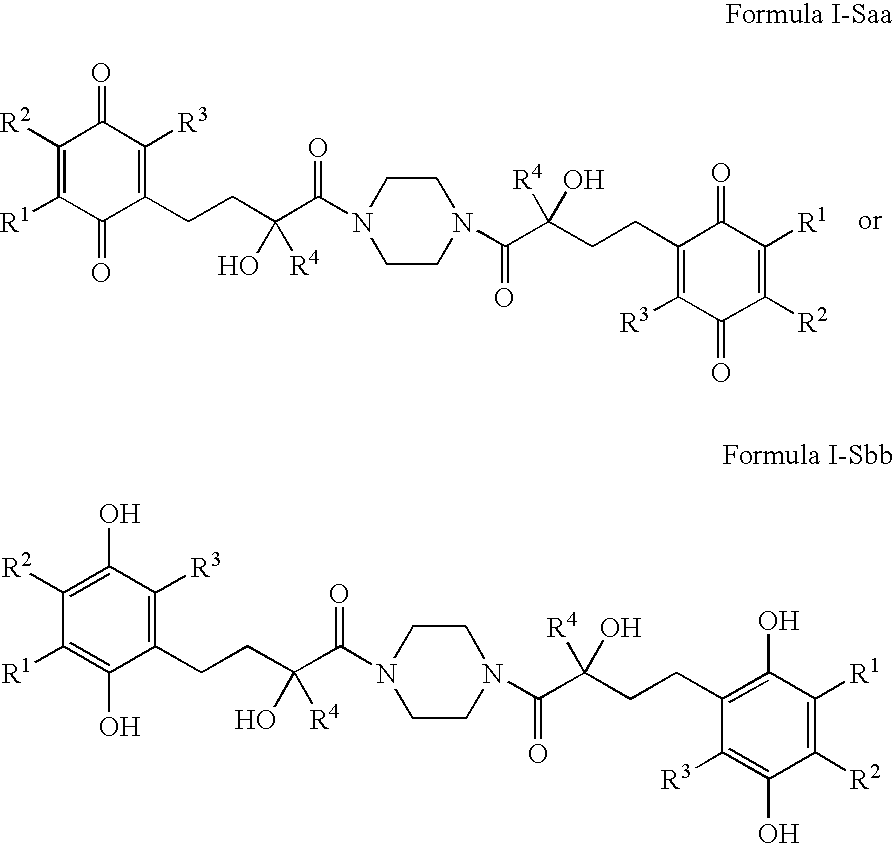
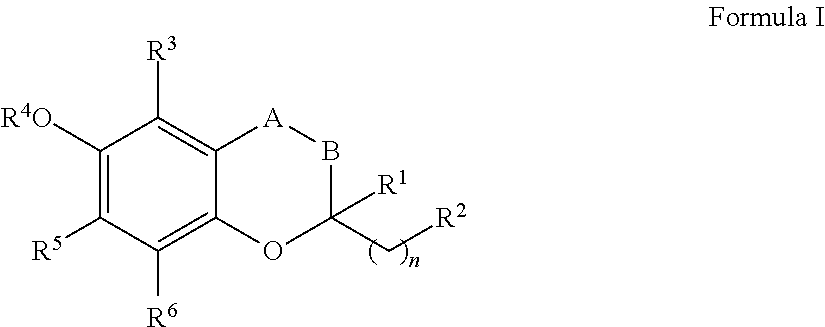
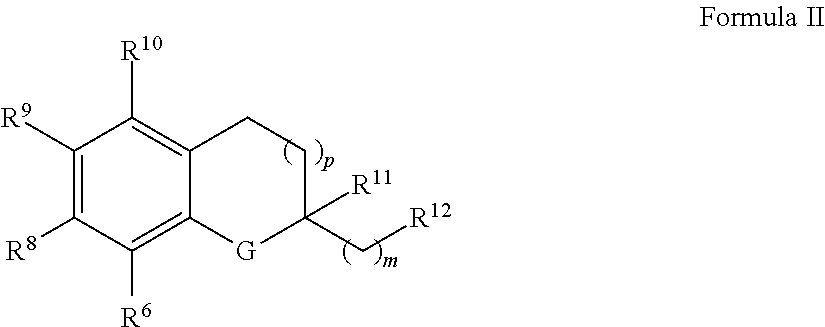
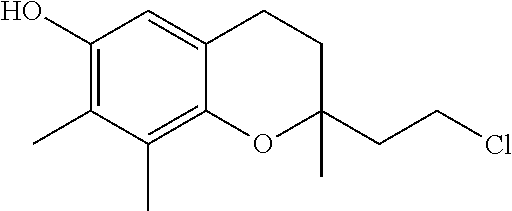
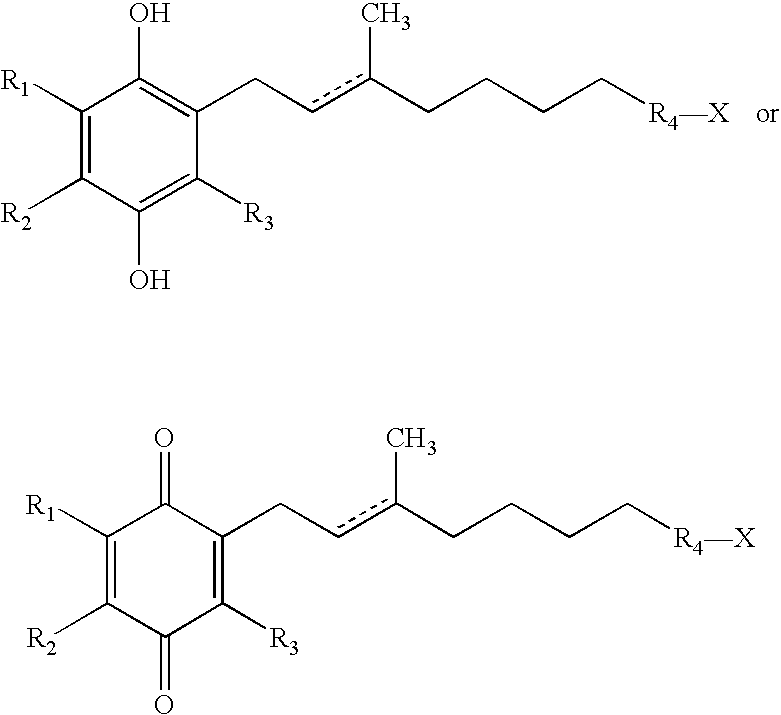
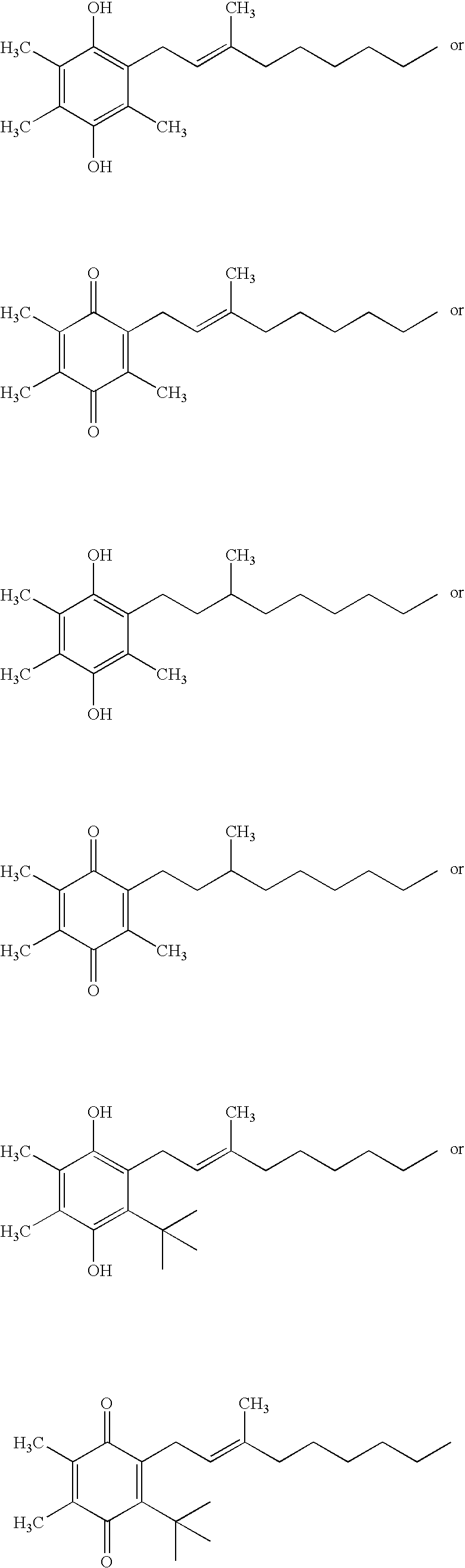
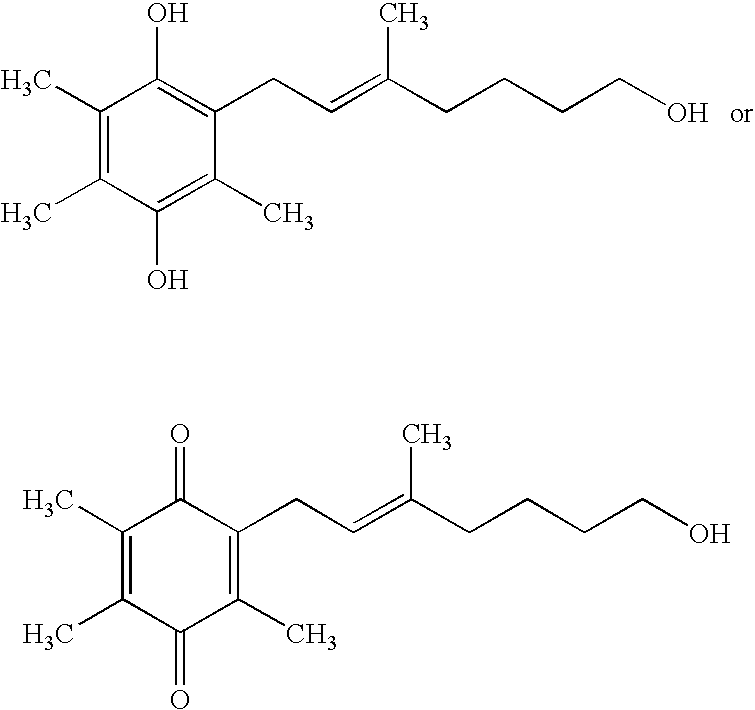
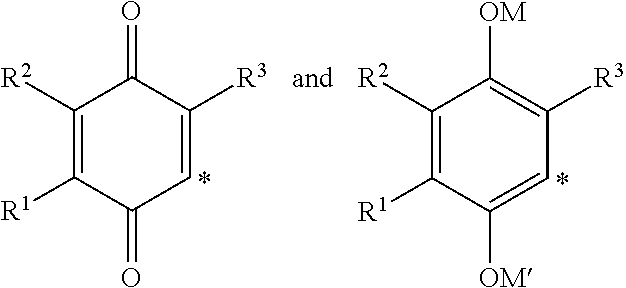
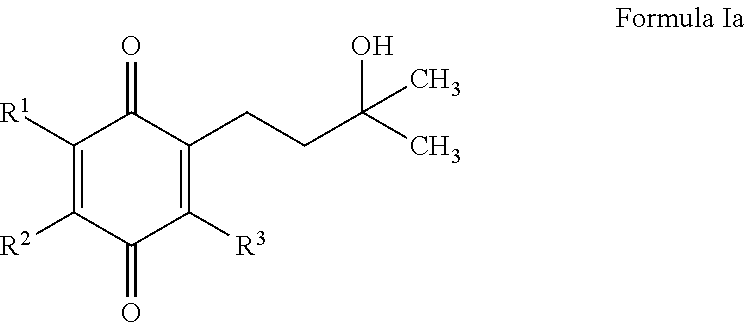
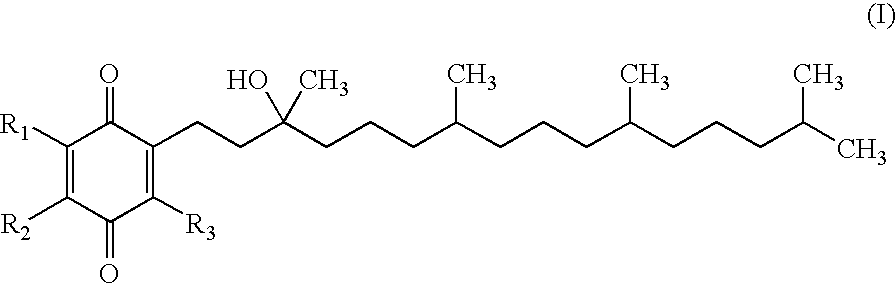
The invention relates the method of treatment or amelioration of mitochondrial disorders such as Alzheimer's disease, Parkinson's disease, Friedreich's ataxia (FRDA), cerebellar ataxias, Leber's hereditary optic neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), Myoclonic Epilepsy with Ragged Red Fibers (MERFF), amyotrophic lateral sclerosis (ALS), motor neuron diseases, Huntington's disease, macular degeneration, and epilepsy, with chroman derivatives of Formula I or Formula II as described herein.
Owner:EDISON PHARMA
Redox-active therapeutics for treatment of mitochondrial diseases and other conditions and modulation of energy biomarkers
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), or Kearns-Sayre Syndrome (KSS) are disclosed, as well as compounds useful in the methods of the invention, such as alpha-tocopherol quinone. Methods and compounds useful in treating other disorders are also disclosed. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed. Methods of modulating, normalizing, or enhancing energy biomarkers, as well as compounds useful for such methods, are also disclosed.
Owner:PTC THERAPEUTICS INC
Side-chain variants of redox-active therapeutics for treatment of mitochondrial diseases and other conditions and modulation of energy biomarkers
InactiveUS20070225261A1Enhancing oneEnhancing more energy biomarkersBiocideSenses disorderDiseaseKearn sayre syndrome
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), or Kearns-Sayre Syndrome (KSS) are disclosed, as well as compounds useful in the methods of the invention. Methods and compounds useful in treating other disorders are also disclosed. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed. Methods of modulating, normalizing, or enhancing energy biomarkers, as well as compounds useful for such methods, are also disclosed.
Owner:PTC THERAPEUTICS INC
Tail variants of redox-active therapeutics for treatment of mitochondrial diseases and other conditions and modulation of energy biomarkers
ActiveUS7432305B2Easy to modifyReduce severityAntibacterial agentsBiocideKearn sayre syndromeMitochondrial myopathy
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), or Kearns-Sayre Syndrome (KSS) are disclosed, as well as compounds useful in the methods of the invention. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed.
Owner:PTC THERAPEUTICS INC
Tail variants of redox-active therapeutics for treatment of mitochondrial diseases and other conditions and modulation of energy biomarkers
ActiveUS20070072943A1Lower Level RequirementsExercise toleranceAntibacterial agentsBiocideKearn sayre syndromeFriedreichs ataxia
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), or Kearns-Sayre Syndrome (KSS) are disclosed, as well as compounds useful in the methods of the invention. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed.
Owner:PTC THERAPEUTICS INC
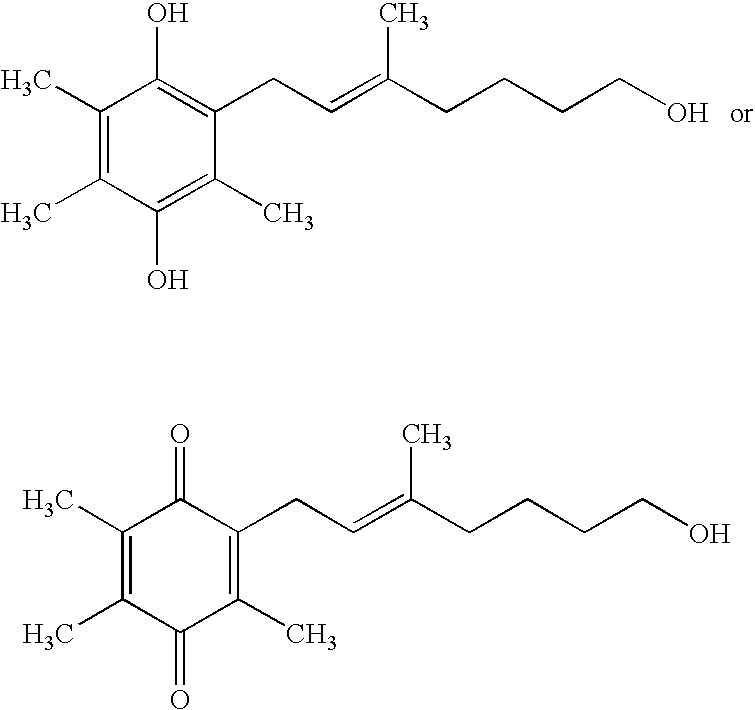
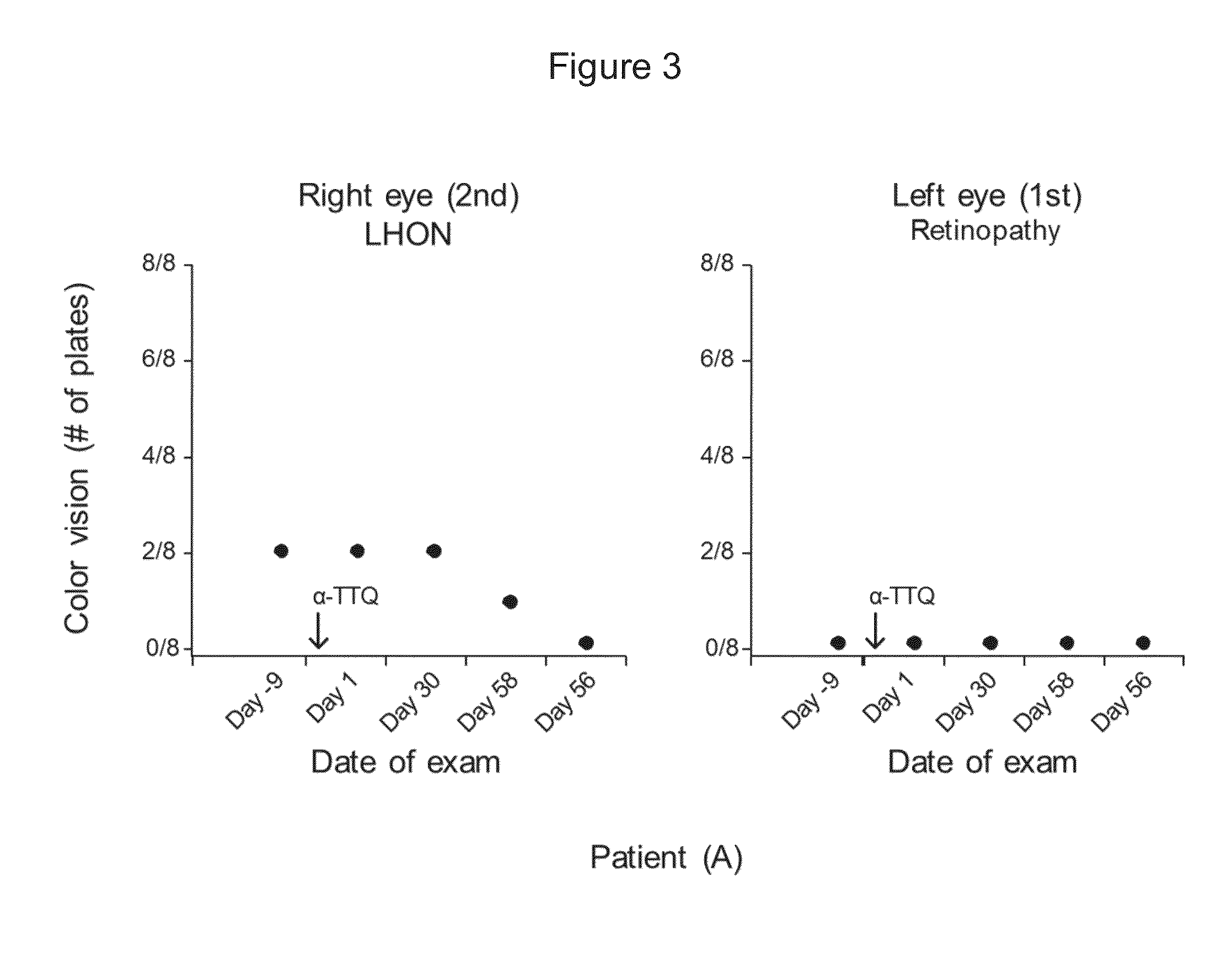
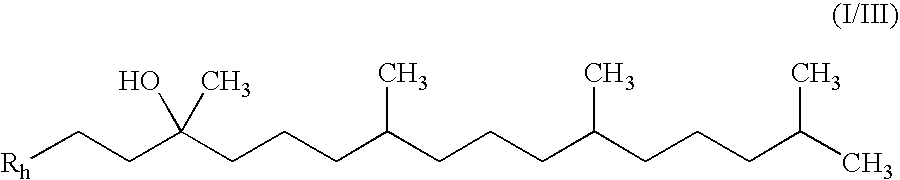
Treatment of leber's hereditary optic neuropathy and dominant optic atrophy with tocotrienol quinones
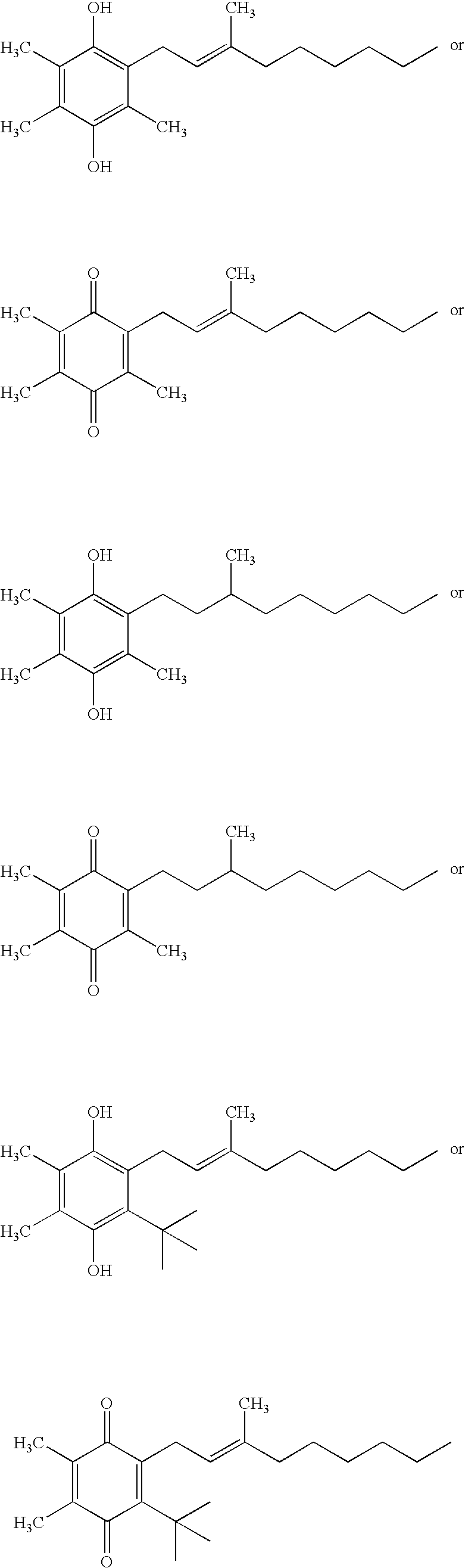
The present invention relates to methods of treating Leber's hereditary optic neuropathy and dominant optic atrophy with tocotrienol quinones, including alpha-tocotrienol quinone, in order to alleviate symptoms of the disease.
Owner:EDISON PHARMA
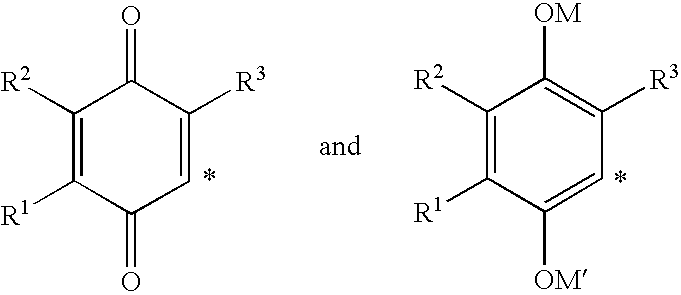
(HET)aryl-p-quinone derivatives for treatment of mitochondrial diseases
InactiveUS20110046219A1Good for healthRaise level of ATPBiocideSenses disorderQuinoneKearn sayre syndrome
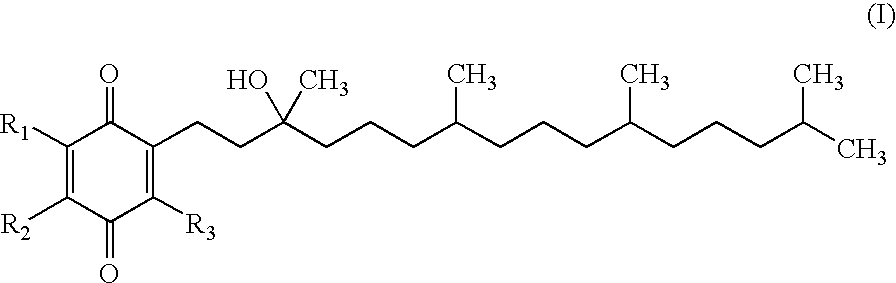
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), Kearns-Sayre Syndrome (KSS), are disclosed, as well as compounds useful in the methods of the invention, such as 2-(3-hydroxy-3-methyl-butyl)-6-(het)aryl-p-quinone or as 2-(3-hydroxy-3-methylbutyl)-3-(het)aryl-p-quinone derivatives. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed. Methods of modulating, normalizing, or enhancing energy biomarkers, as well as compounds useful for such methods, are also disclosed.
Owner:PTC THERAPEUTICS INC
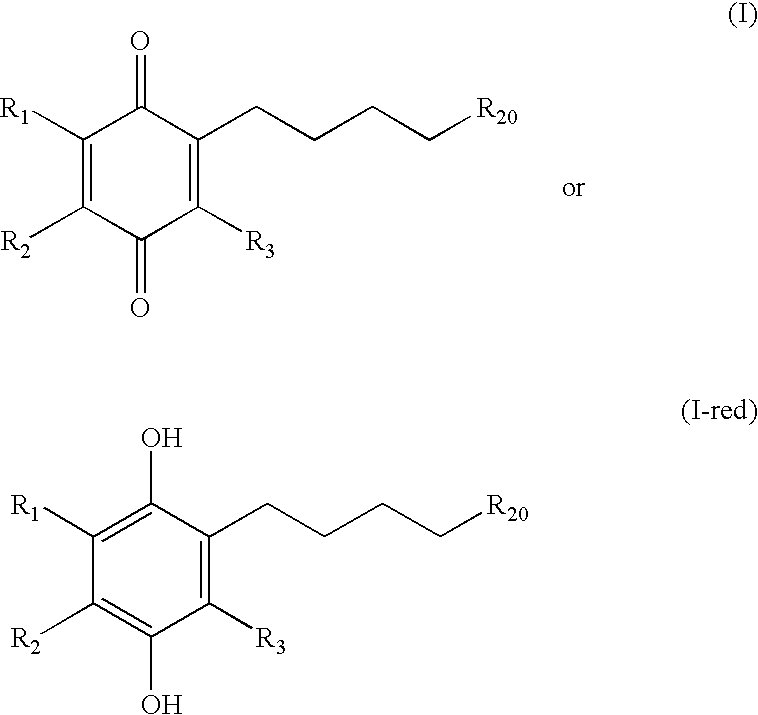
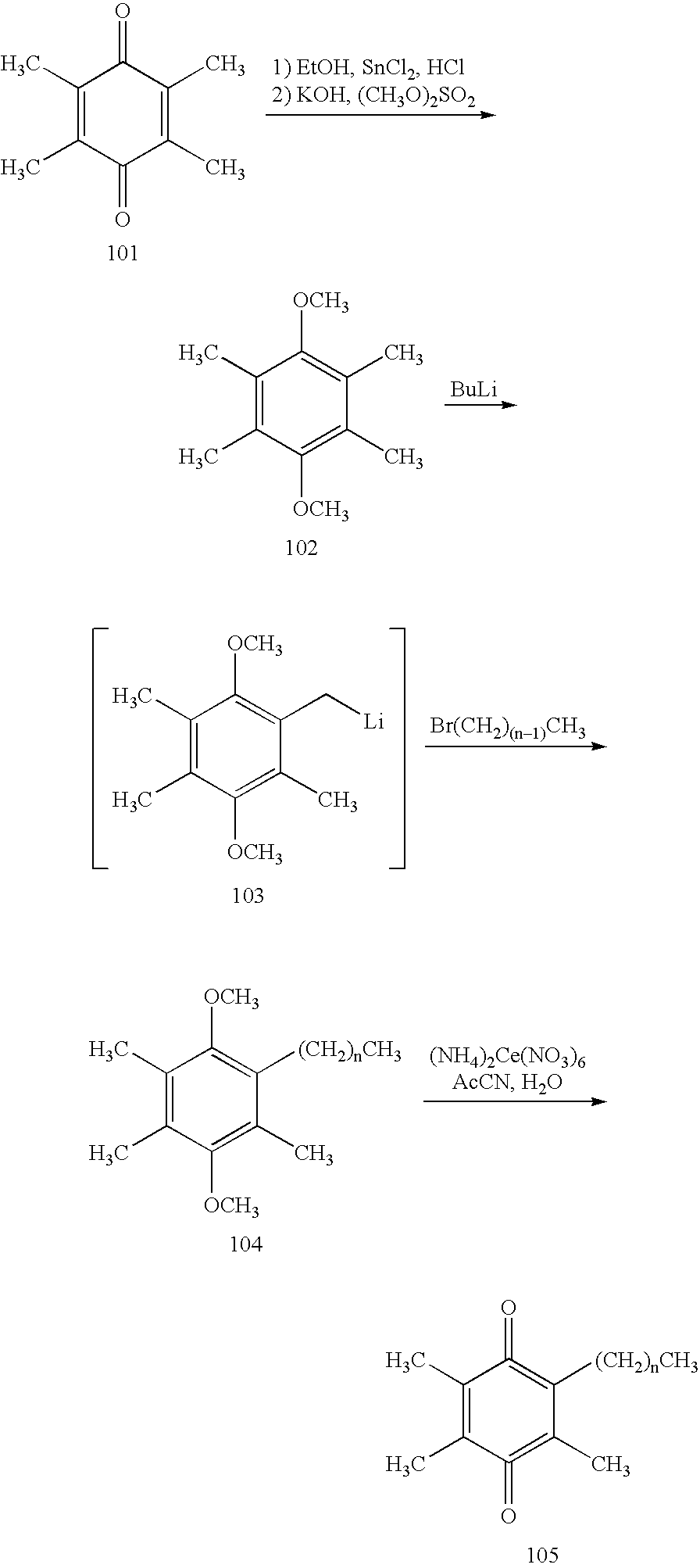
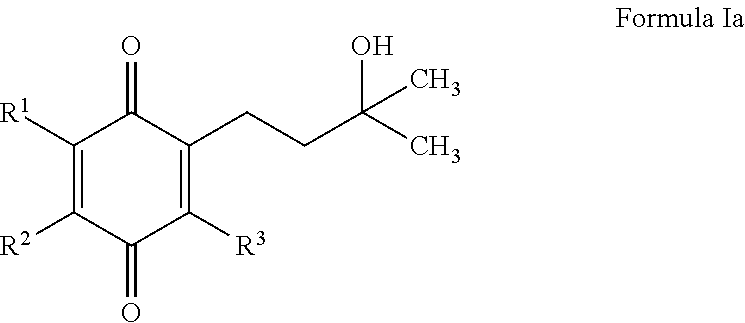
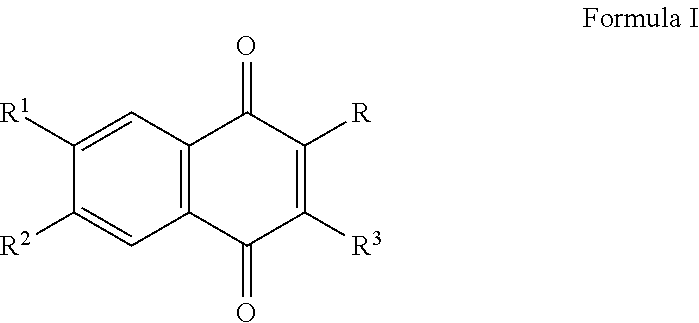
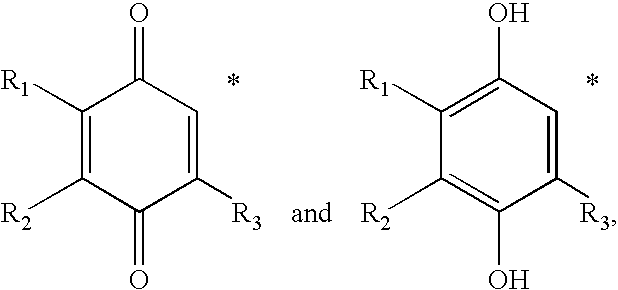
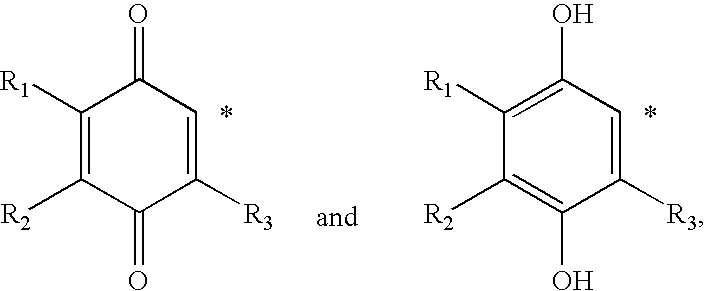
Treatment of mitochondrial diseases with naphthoquinones
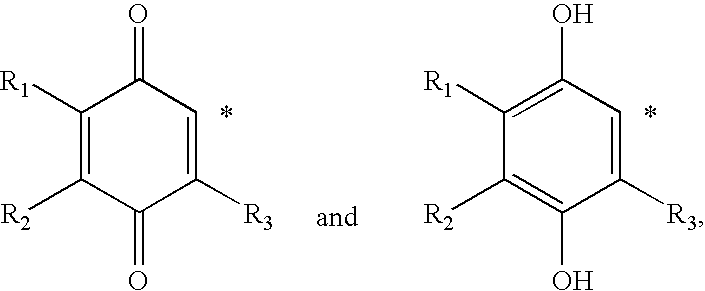
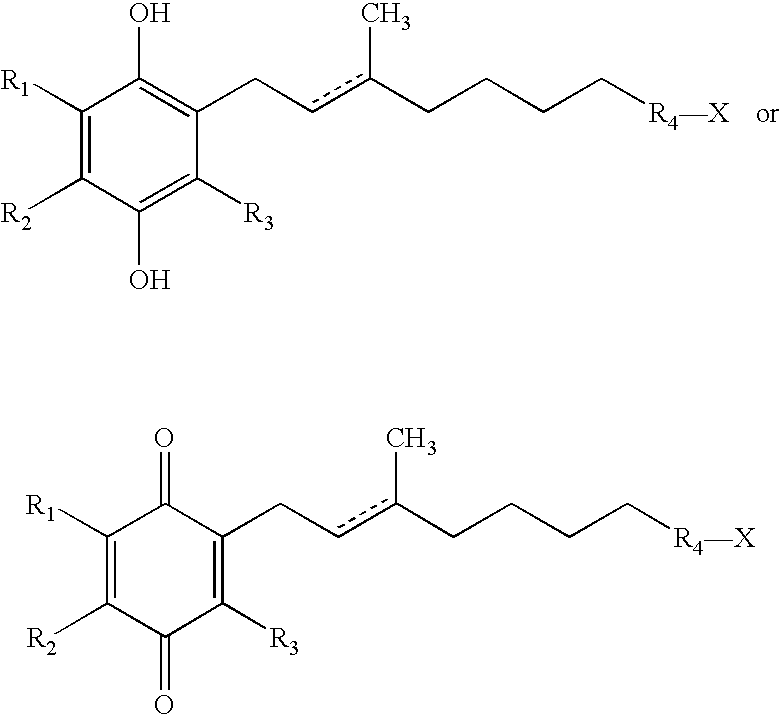
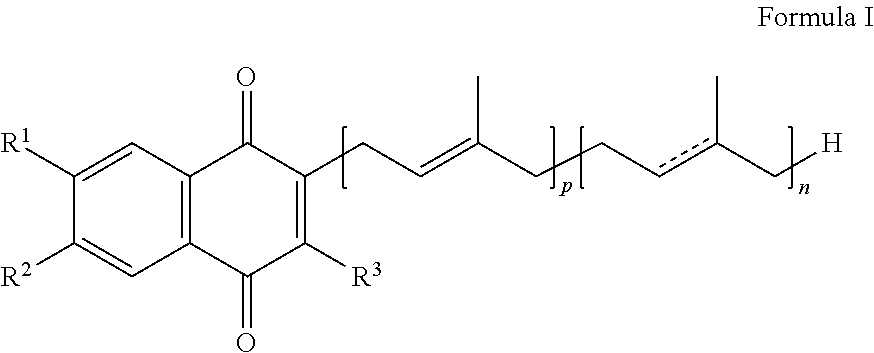
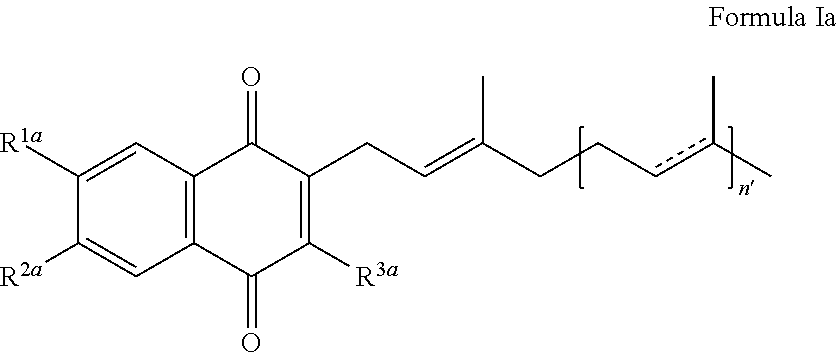
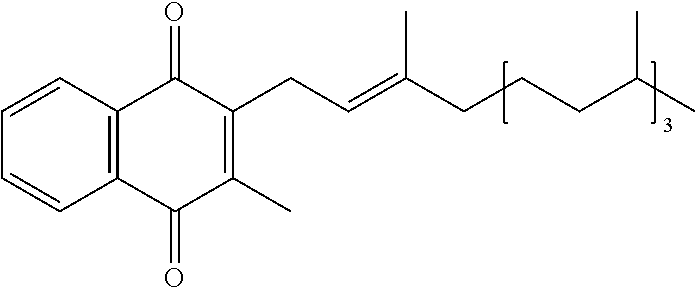
Methods of treating, preventing or suppressing symptoms associated with mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), dominant optic atrophy (DOA); mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), Leigh syndrome or Kearns-Sayre Syndrome (KSS) with compounds of Formula (I) are disclosed. Methods of modulating, normalizing, or enhancing energy biomarkers, as well as compounds useful for such methods are also disclosed.
Owner:BIOELECTRON TECH CORP
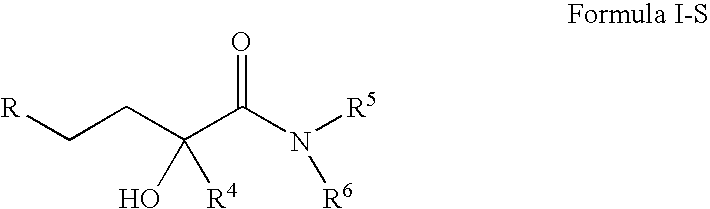
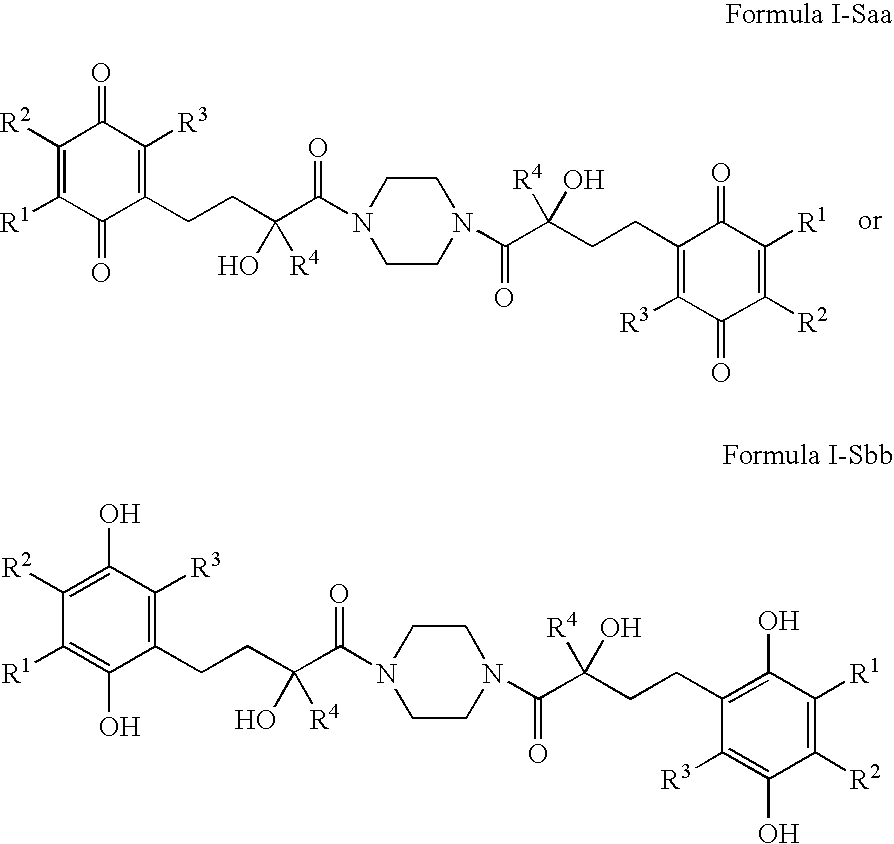
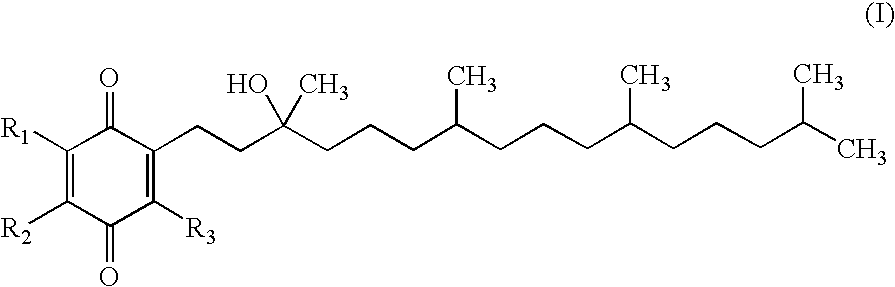
4-(p-QUINONYL)-2-HYDROXYBUTANAMIDE DERIVATIVES FOR TREATMENT OF MITOCHONDRIAL DISEASES
ActiveUS20090118257A1Good for healthRaise level of ATPBiocideSenses disorderKearn sayre syndromeHuntingtons chorea
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, and stroke (MELAS), Kearns-Sayre Syndrome (KSS), are disclosed, as well as compounds useful in the methods of the invention, such as 4-(p-quinolyl)-2-hydroxybutanamide derivatives. Methods and compounds useful in treating other disorders such as amyotrophic lateral sclerosis (ALS), Huntington's disease, Parkinson's disease, and pervasive developmental disorders such as autism are also disclosed. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed. Methods of modulating, normalizing, or enhancing energy biomarkers, as well as compounds useful for such methods, are also disclosed.
Owner:PTC THERAPEUTICS INC
Redox-active therapeutics for treatment of mitochondrial diseases and other conditions and modulation of energy biomarkers
ActiveUS20100222436A1Reduce severityReduce in quantityBiocideSenses disorderDiseaseKearn sayre syndrome
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), or Kearns-Sayre Syndrome (KSS) are disclosed, as well as compounds useful in the methods of the invention, such as alpha-tocopherol quinone. Methods and compounds useful in treating other disorders are also disclosed. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed. Methods of modulating, normalizing, or enhancing energy biomarkers, as well as compounds useful for such methods, are also disclosed.
Owner:PTC THERAPEUTICS INC
4-(p-quinonyl)-2-hydroxybutanamide derivatives for treatment of mitochondrial diseases
ActiveUS7968746B2Reduce severityReduce in quantitySenses disorderNervous disorderHuntingtons choreaKearn sayre syndrome
Owner:PTC THERAPEUTICS INC
Treatment of mitochondrial diseases with vitamin k
InactiveUS20140031432A1Lower Level RequirementsExercise toleranceBiocideSenses disorderDiseaseKearn sayre syndrome
Methods of treating, preventing or suppressing symptoms associated with mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), dominant optic atrophy (DOA); mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), Leigh syndrome or Kearns-Sayre Syndrome (KSS) with vitamin K are disclosed.
Owner:AMPERE LIFE SCI
Treatment of mitochondrial diseases
The invention relates the method of treatment or amelioration of mitochondrial disorders such as Alzheimer's disease, Parkinson's disease, Friedreich's ataxia (FRDA), cerebellar ataxias, Leber's hereditary optic neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), Myoclonic Epilepsy with Ragged Red Fibers (MERFF), amyotrophic lateral sclerosis (ALS), motor neuron diseases, Huntington's disease, macular degeneration, and epilepsy, with chroman derivatives of Formula I or Formula II as described herein.
Owner:EDISON PHARMA
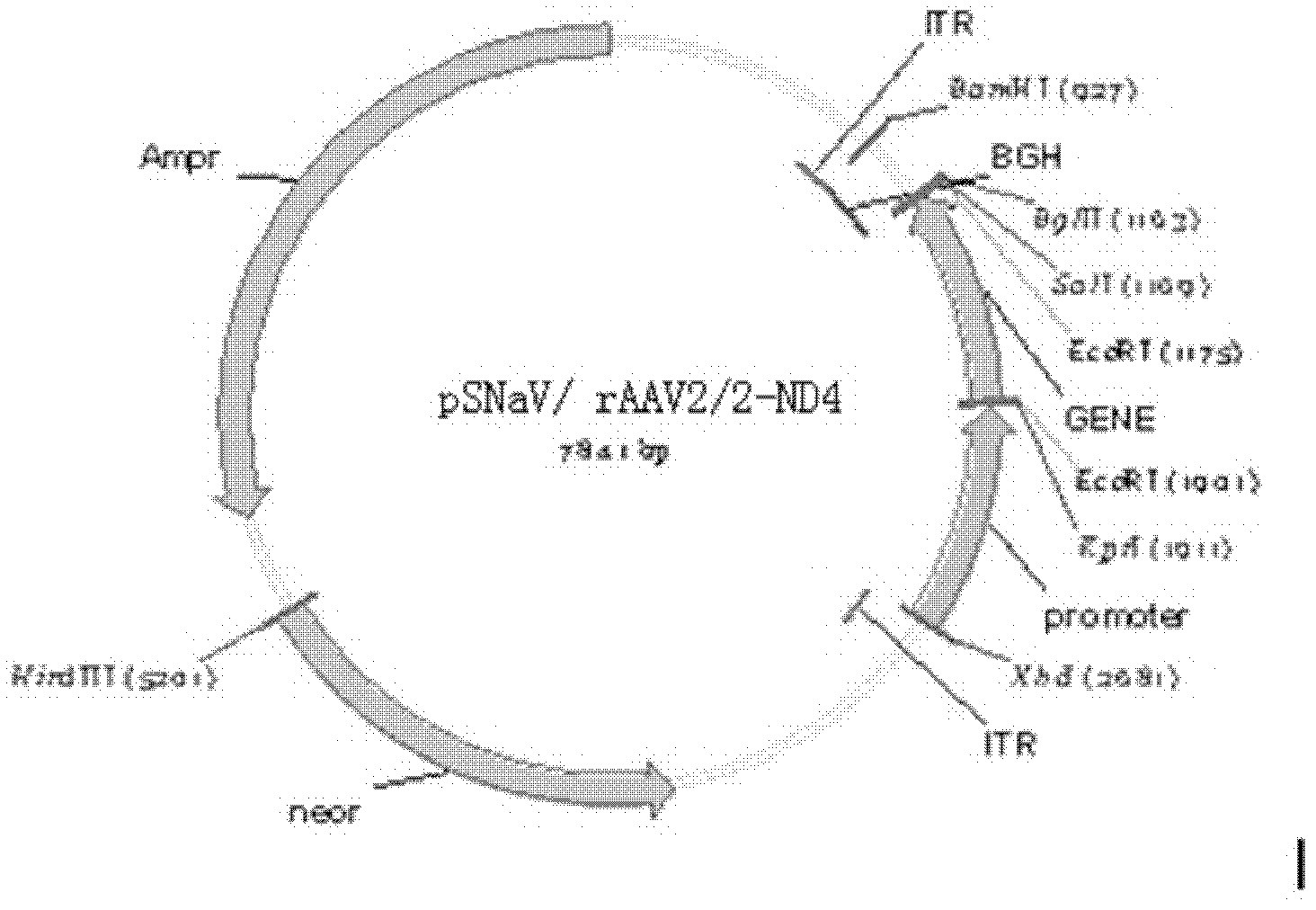
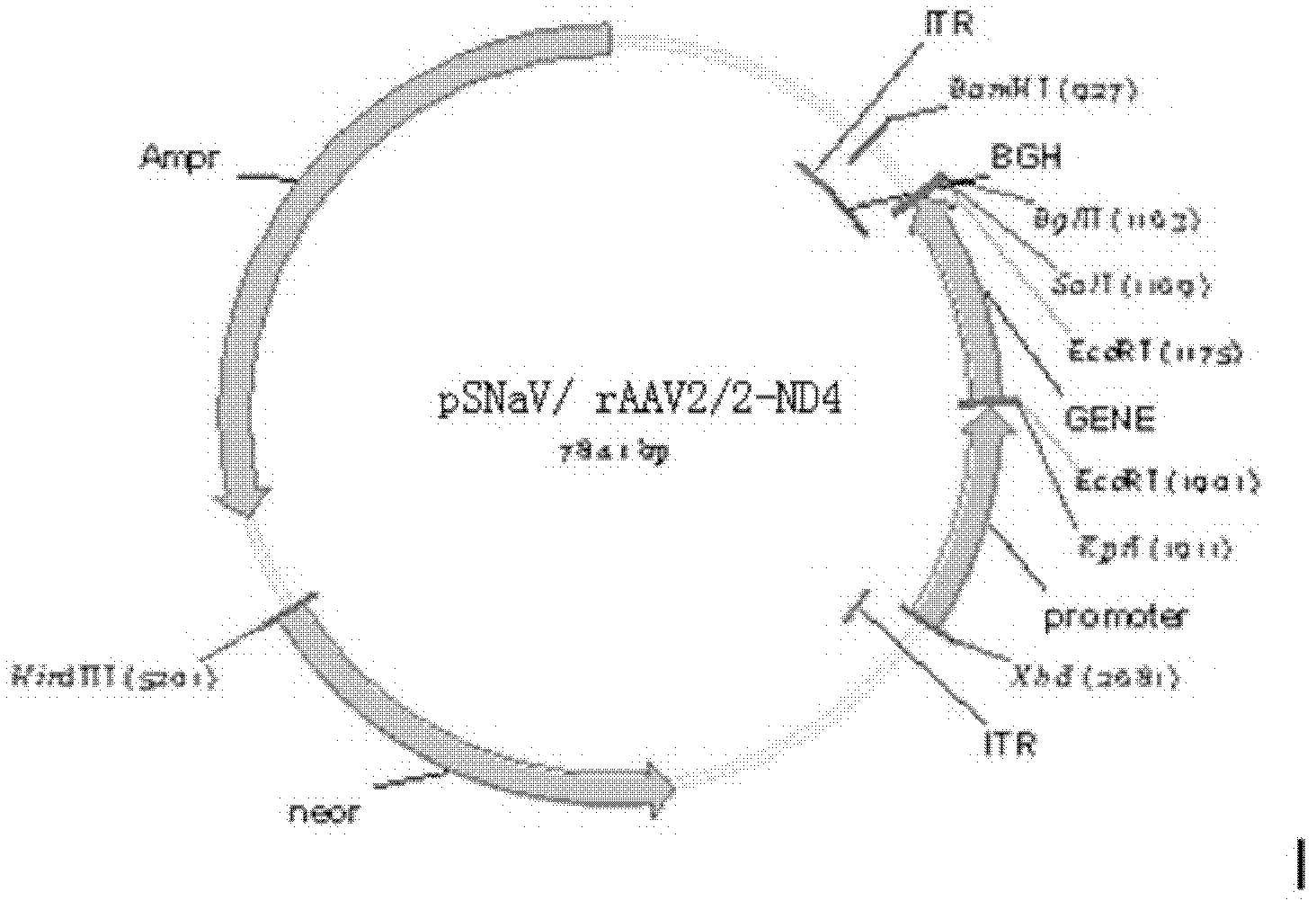
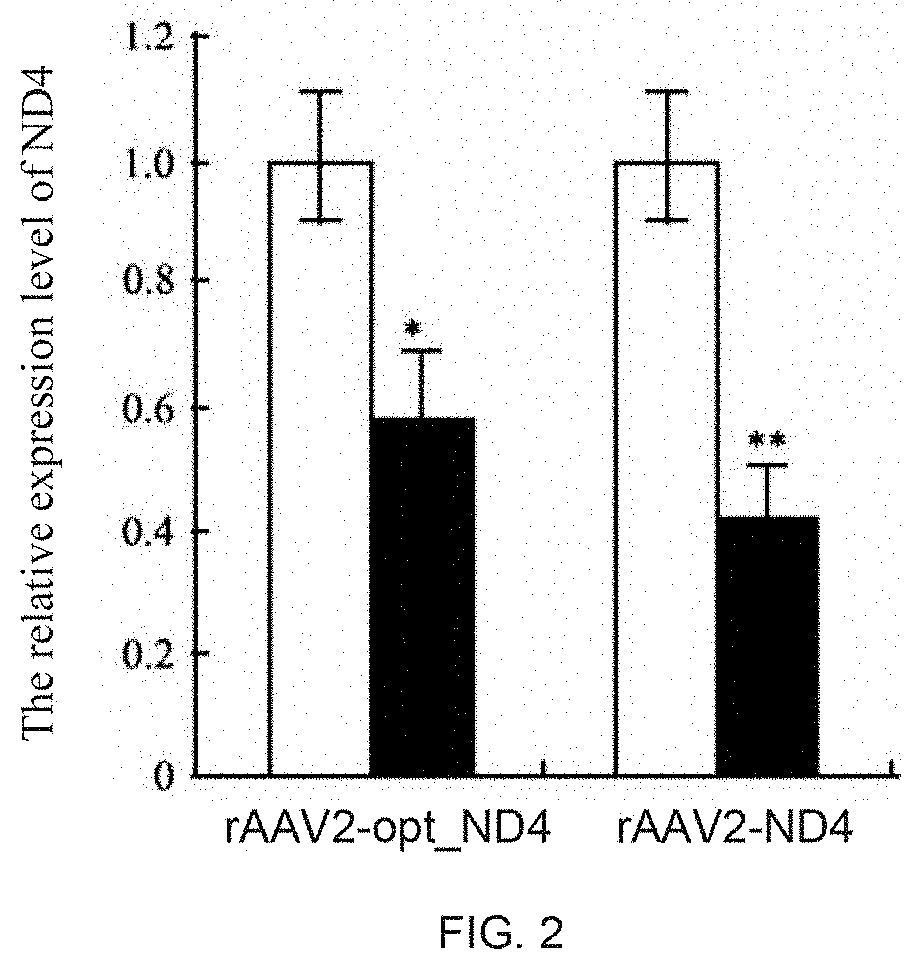
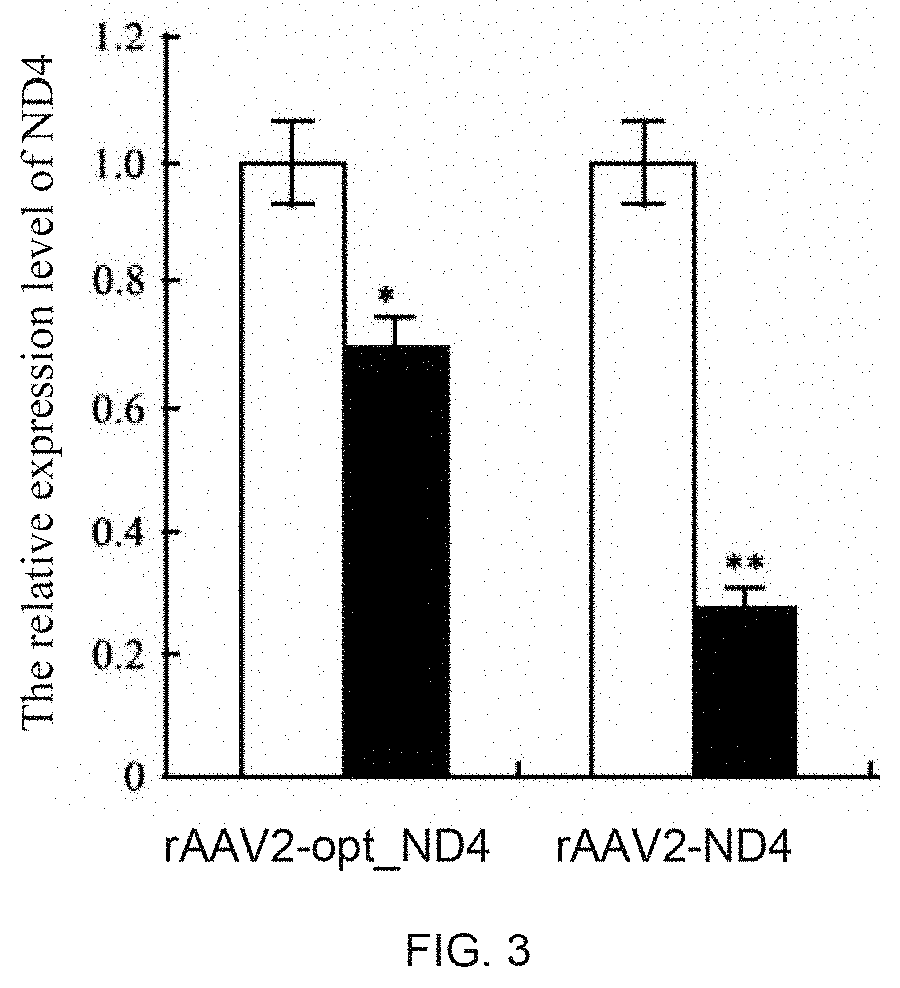
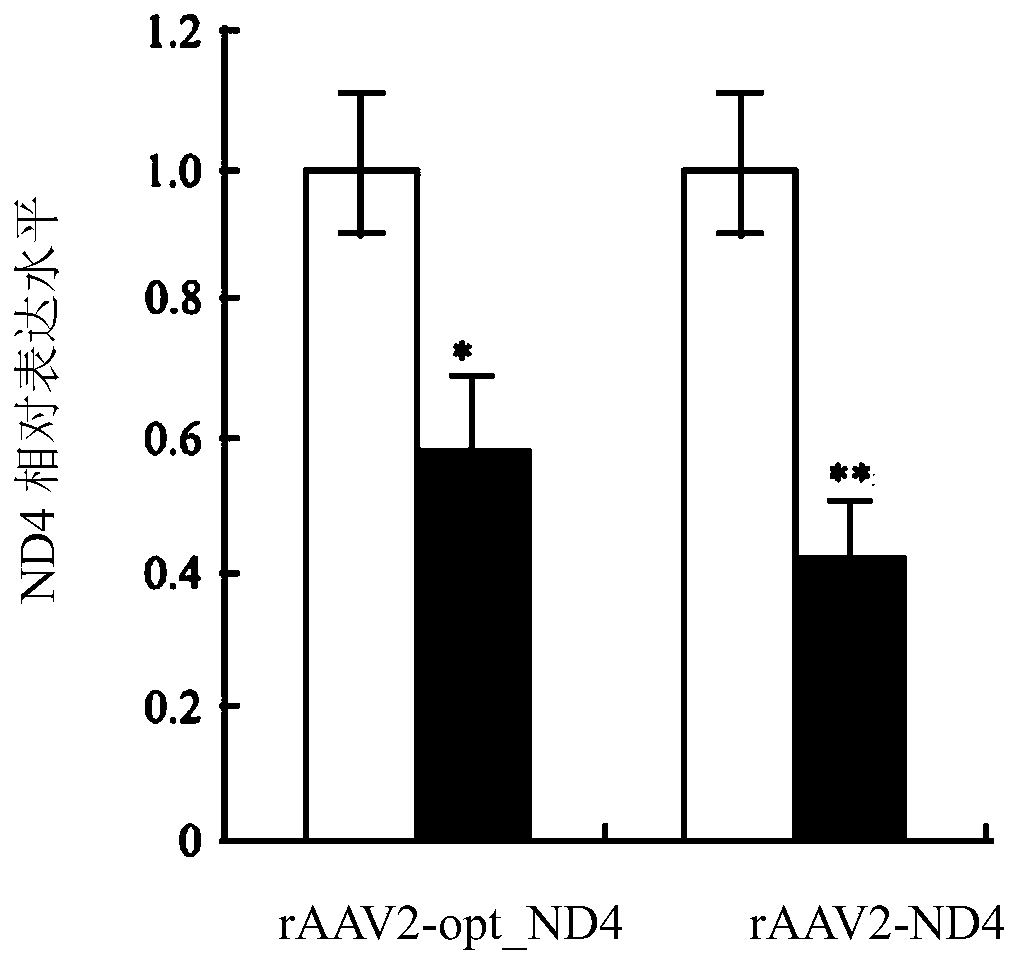
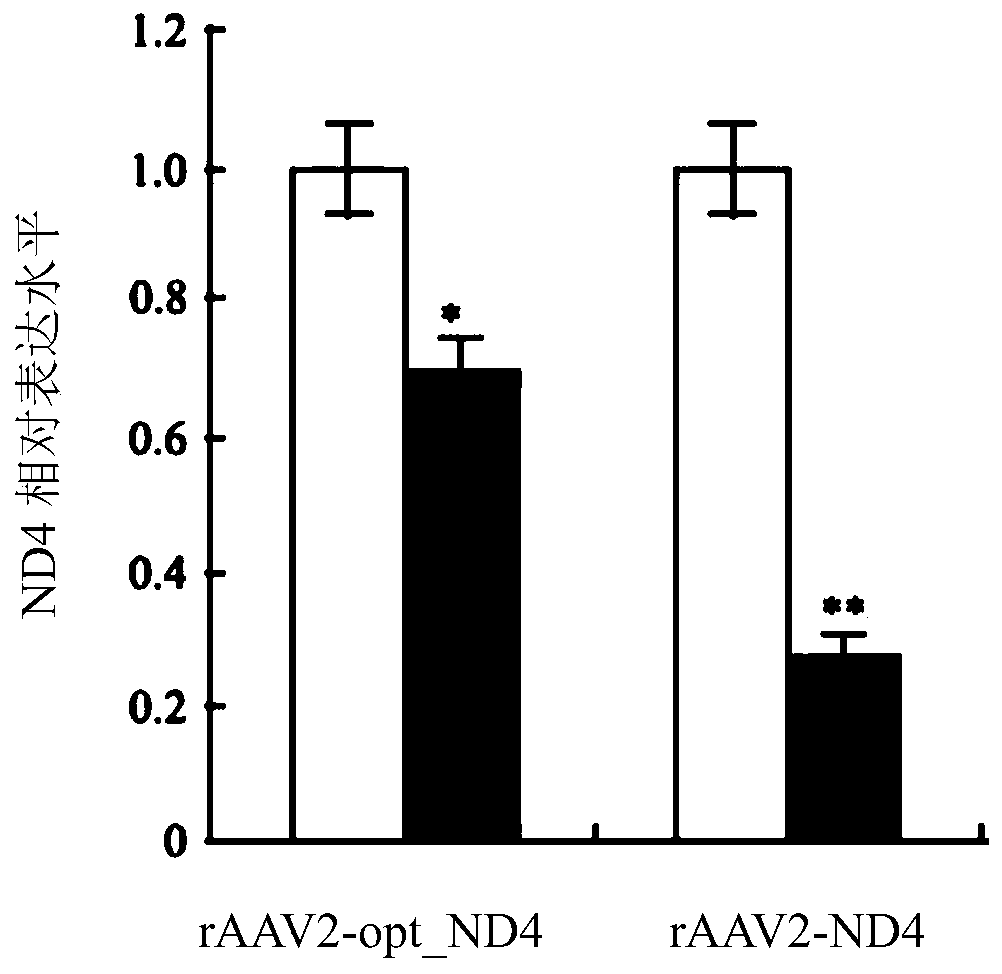
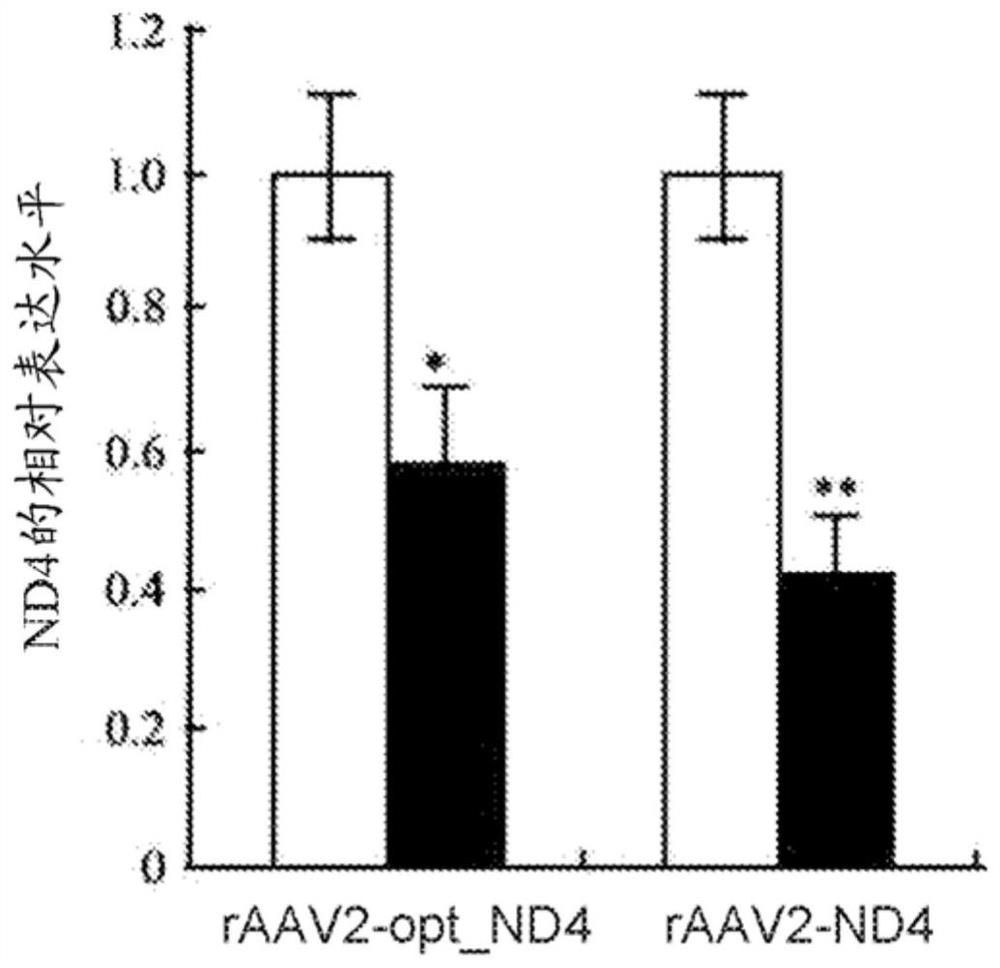
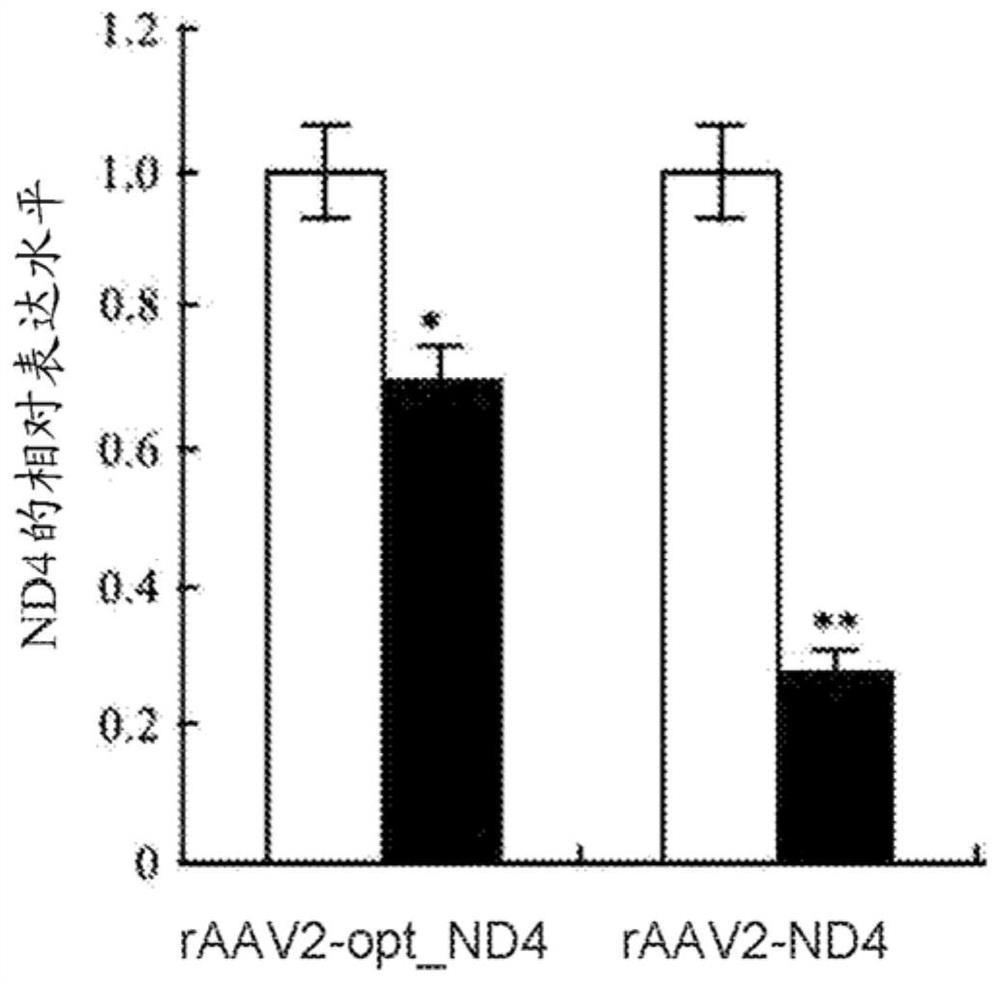
Recombinant human NADH (nicotinamide-adenine dinucleotide) dehydrogenase subunit-4 gene and constructing method of expression vector thereof
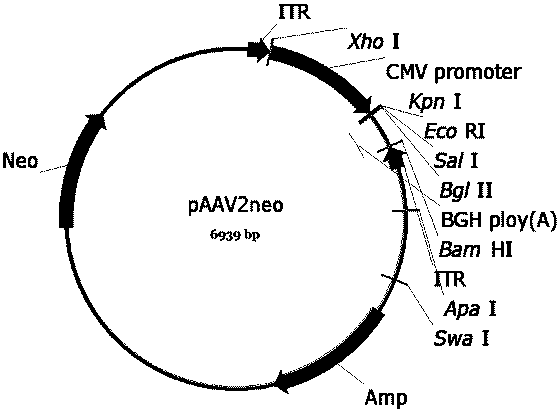
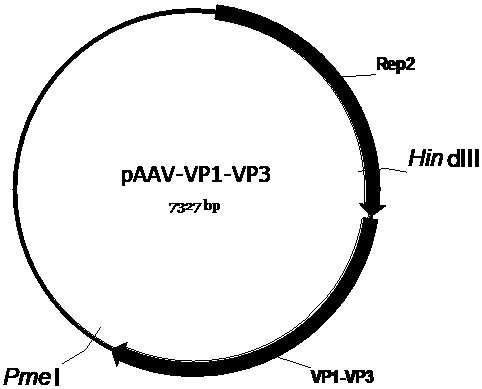
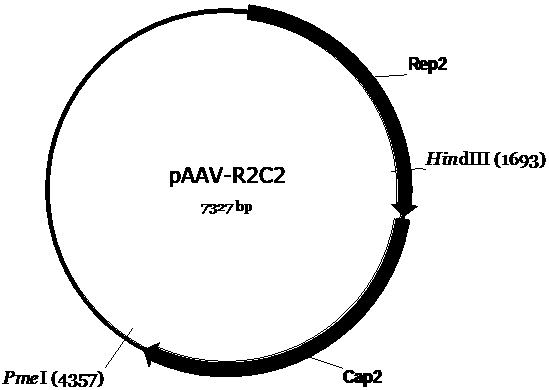
The invention discloses a recombinant human NADH (nicotinamide-adenine dinucleotid) dehydrogenase subunit-4 gene and a constructing method of an expression vector thereof. The nucleotide sequence of the gene is shown in SEQ ID NO:1, and the size of the nucleotide sequence is 2889 bp. The constructing steps of adeno-associate virus vectors are as follows: firstly constructing a recombinant adeno-associated virus vector containing the human NADH dehydrogenase subunit-4 gene, and then coating, infecting, purifying, concentrating and identifying the recombinant adeno-associated virus. According to the method, the recombinant adeno-associated virus vector with the recombinant human NADH dehydrogenase subunit-4 gene can be quickly and simply constructed, and is packaged to obtain the adeno-associated virus vector with complex defects. The gene can be used for gene therapy of LHON (leber's hereditary optic neuropathy).
Owner:WUHAN NEUROPHTH BIOTECHNOLOGY LTD CO
Treatment of leber's hereditary optic neuropathy and dominant optic atrophy with tocotrienol quinones
ActiveUS20140039065A1Stops progression of loss of color visionStop progressBiocideSenses disorderDiseaseAtrophy
The present invention relates to methods of treating Leber's hereditary optic neuropathy and dominant optic atrophy with tocotrienol quinones, including alpha-tocotrienol quinone, in order to alleviate symptoms of the disease.
Owner:PTC THERAPEUTICS INC
Recombinant human NADH (nicotinamide-adenine dinucleotide) dehydrogenase subunit-4 gene and constructing method of expression vector thereof
Owner:WUHAN NEUROPHTH BIOTECHNOLOGY LTD CO
Side-chain variants of redox-active therapeutics for treatment of mitochondrial diseases and other conditions and modulation of energy biomarkers
InactiveUS9278085B2Reduce severityReduce in quantityBiocideSenses disorderDiseaseKearn sayre syndrome
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), or Kearns-Sayre Syndrome (KSS) are disclosed, as well as compounds useful in the methods of the invention. Methods and compounds useful in treating other disorders are also disclosed. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed. Methods of modulating, normalizing, or enhancing energy biomarkers, as well as compounds useful for such methods, are also disclosed.
Owner:PTC THERAPEUTICS INC
(Het)aryl-p-quinone derivatives for treatment of mitochondrial diseases
InactiveUS8952071B2Reduce severityReduce in quantityBiocideSenses disorderQuinoneKearn sayre syndrome
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), Kearns-Sayre Syndrome (KSS), are disclosed, as well as compounds useful in the methods of the invention, such as 2-(3-hydroxy-3-methyl-butyl)-6-(het)aryl-p-quinone or as 2-(3-hydroxy-3-methylbutyl)-3-(het)aryl-p-quinone derivatives. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed. Methods of modulating, normalizing, or enhancing energy biomarkers, as well as compounds useful for such methods, are also disclosed.
Owner:PTC THERAPEUTICS INC
Regulation of receptor expression through delivery of artificial transcription factors
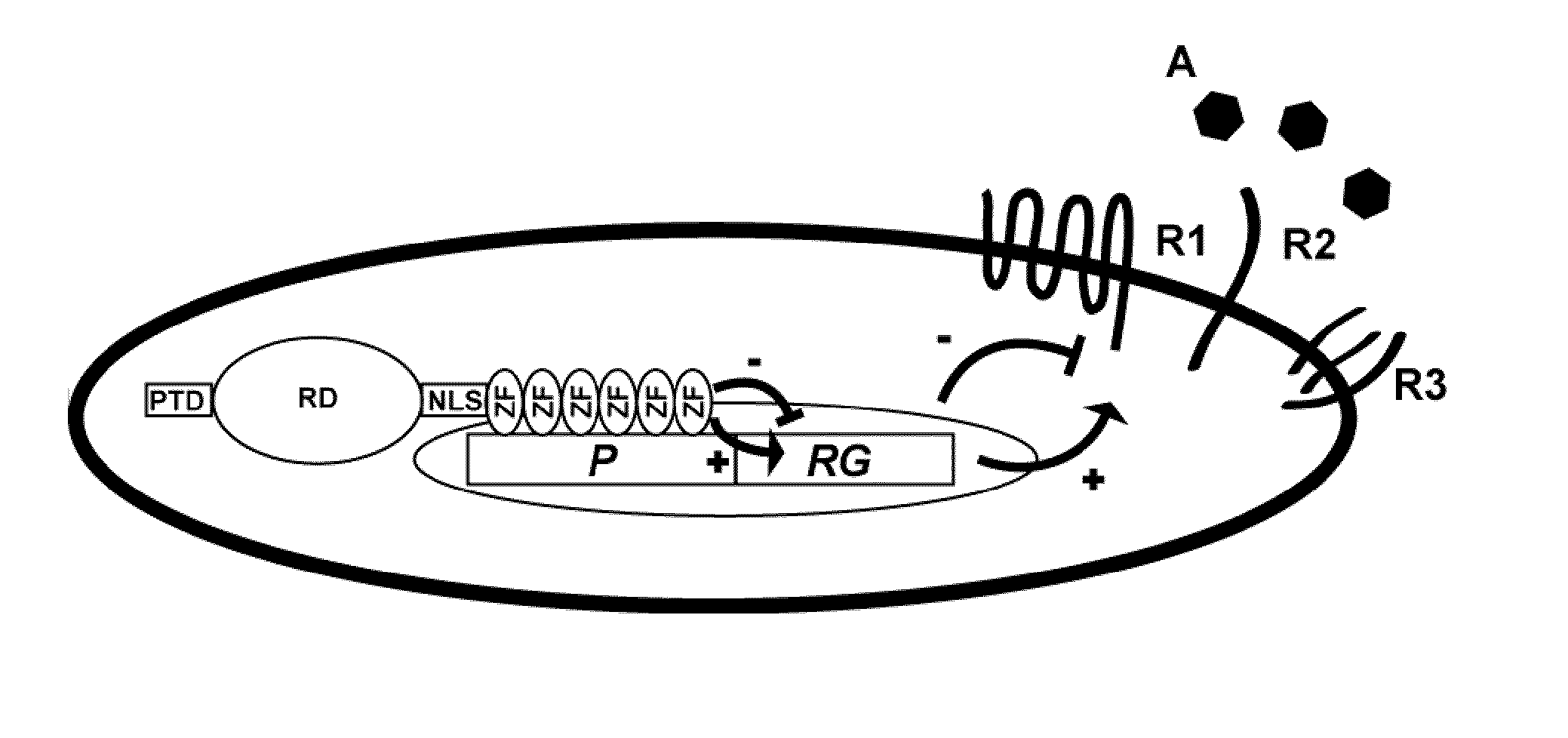
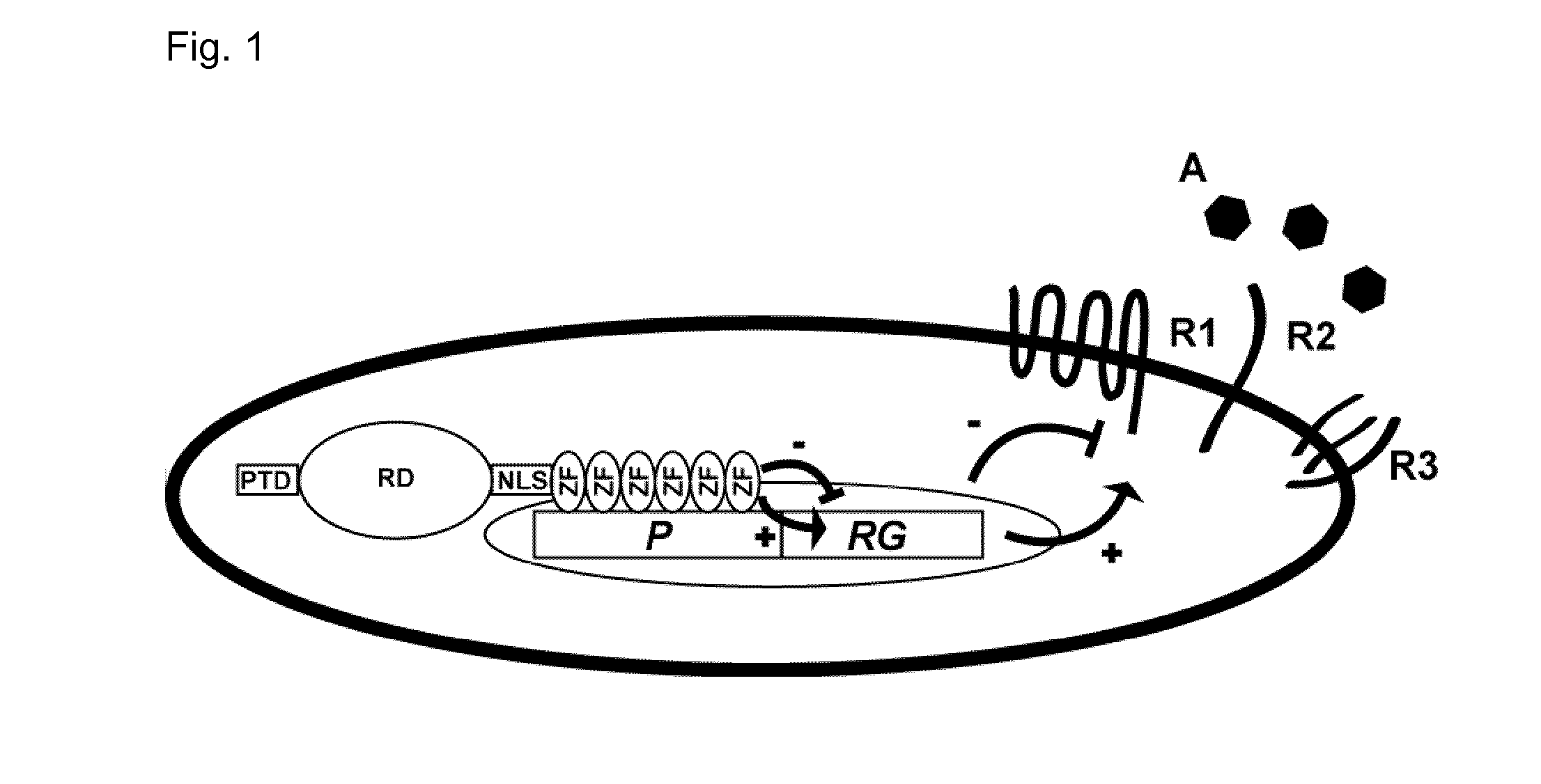
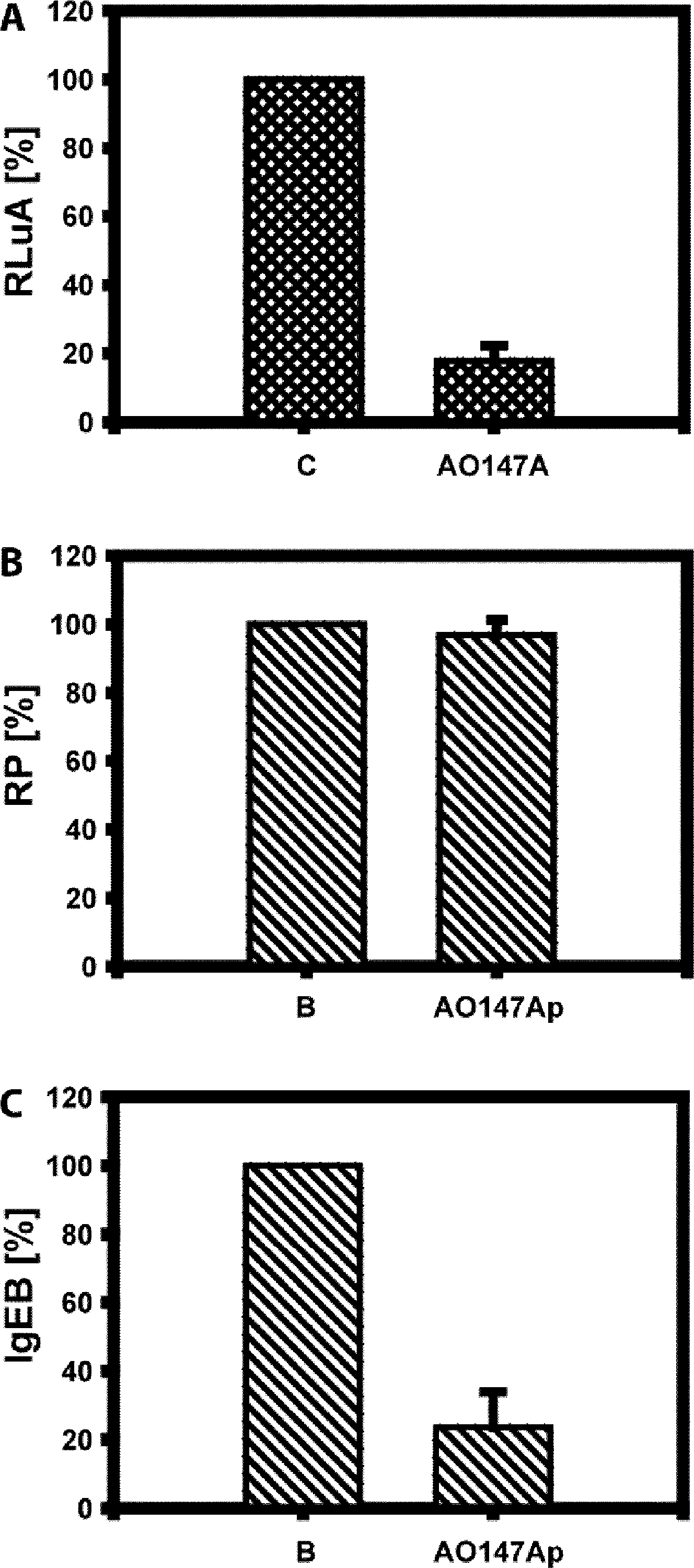
The invention relates to an artificial transcription factor comprising a polydactyl zinc finger protein targeting specifically a receptor gene promoter fused to an inhibitory or activatory protein domain, a nuclear localization sequence, and a protein transduction domain. In particular examples these receptor gene promoters regulate the expression of the endothelin receptor A, the endothelin receptor B, the Toll-like receptor 4 or the high-affinity IgE receptor. Artificial transcription factors directed to the endothelin A or B receptors are useful in the treatment of diseases modulated by endothelin, such as cardiovascular diseases, and, in particular, eye diseases, e.g. retinal vein occlusion, retinal artery occlusion, macular edema, optic neuropathy, central serous chorioretinopathy, retinitis pigmentosa, Leber's hereditary optic neuropathy, and the like. Artificial transcription factors directed to the Toll-like receptor 4 or the IgE receptor are useful for the treatment of autoimmune disorders, and the like, and allergic disorders, respectively.
Owner:ALIOPHTHA
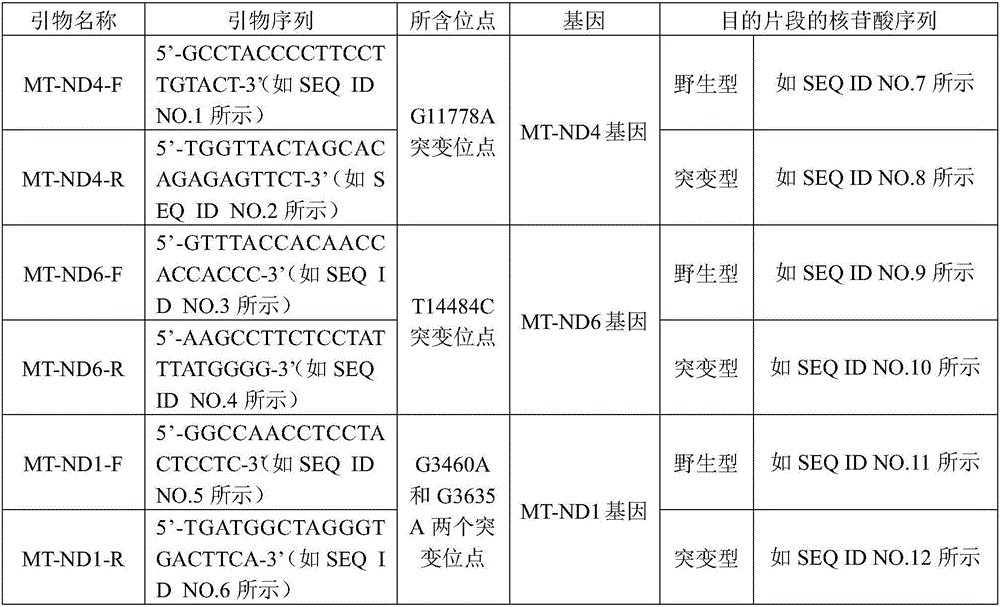
In-vitro diagnostic kit of Leber hereditary optic neuropathy
ActiveCN103981255AEliminate cross-effectsNothing producedMicrobiological testing/measurementPremarital counselingLEBER HEREDITARY OPTIC NEUROPATHY
The invention provides an in-vitro diagnostic kit of Leber hereditary optic neuropathy (LHON). The in-vitro diagnostic kit comprises SEQIDNO.1-12. The in-vitro diagnostic kit provides a rapid, accurate and cheap genetic level detection means for the clinic diagnosis of the LHON, can realize the prevention and early-stage timely diagnosis of the LHON, can find high risk group, improves the diagnosis rate of the HLON, can effectively realize antenatal genetic counseling and premarital counseling, improves the population quality, and reduces the family and national medical expenditure.
Owner:CHILDRENS HOSPITAL OF CHONGQING MEDICAL UNIV +1
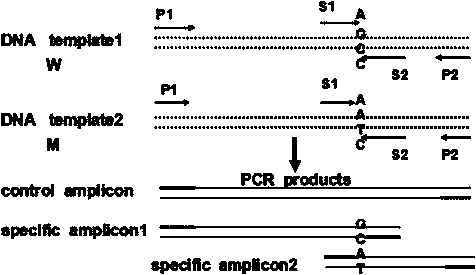
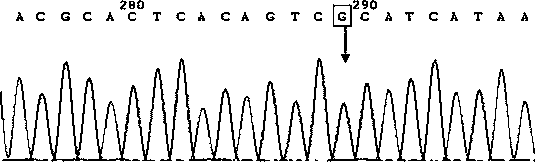
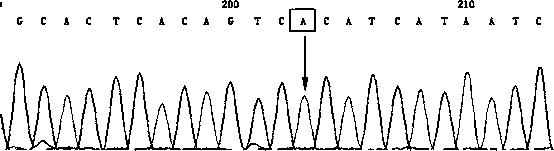
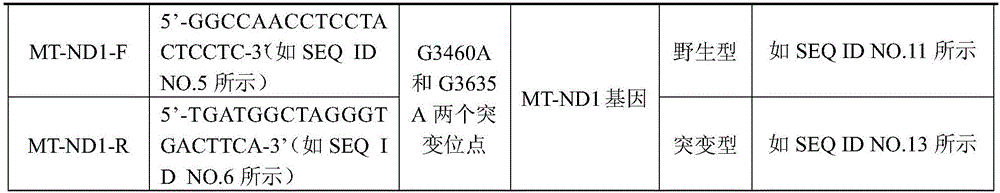
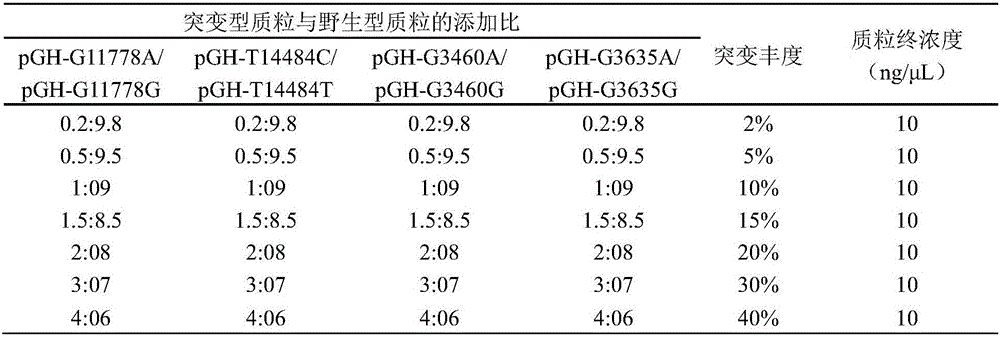
Detection primer, detection kit and detection method of Leber's hereditary optic neuropathy (LHON) mitochondrial DNA gene mutations
ActiveCN106755335AResolve SensitivitySolve the characteristicsMicrobiological testing/measurementDNA/RNA fragmentationGenetic dnaGene mutation
The invention relates to a detection primer, a detection kit and a detection method of Leber's hereditary optic neuropathy (LHON) mitochondrial DNA gene mutations. The invention, on the basis of Sanger sequencing principle, has the characteristics of being highly sensitive, stable and accurate; four most common pathogenic mutations of LHON mtDNA can be simultaneously detected, so that the problems of an existing LHON mitochondrial DNA mutation detection method, which is low in sensitivity, not strong enough in specificity, capable of causing pollution easily, high in expense and the like, can be solved; therefore, an accurate gene diagnosis method is provided for LHON; and the invention has important implications for genetic counseling and gene therapy of the disease (the LHON).
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
Redox-active therapeutics for treatment of mitochondrial diseases and other conditions and modulation of energy biomarkers
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), or Kearns-Sayre Syndrome (KSS) are disclosed, as well as compounds useful in the methods of the invention, such as alpha-tocopherol quinone. Methods and compounds useful in treating other disorders are also disclosed. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed. Methods of modulating, normalizing, or enhancing energy biomarkers, as well as compounds useful for such methods, are also disclosed.
Owner:PTC THERAPEUTICS INC
Gene detection method of Leber's hereditary optic neuropathy, (LHON) gene chip and kit
ActiveCN104805210AShorten the timeRelieve painMicrobiological testing/measurementOptic nerveMitophagy
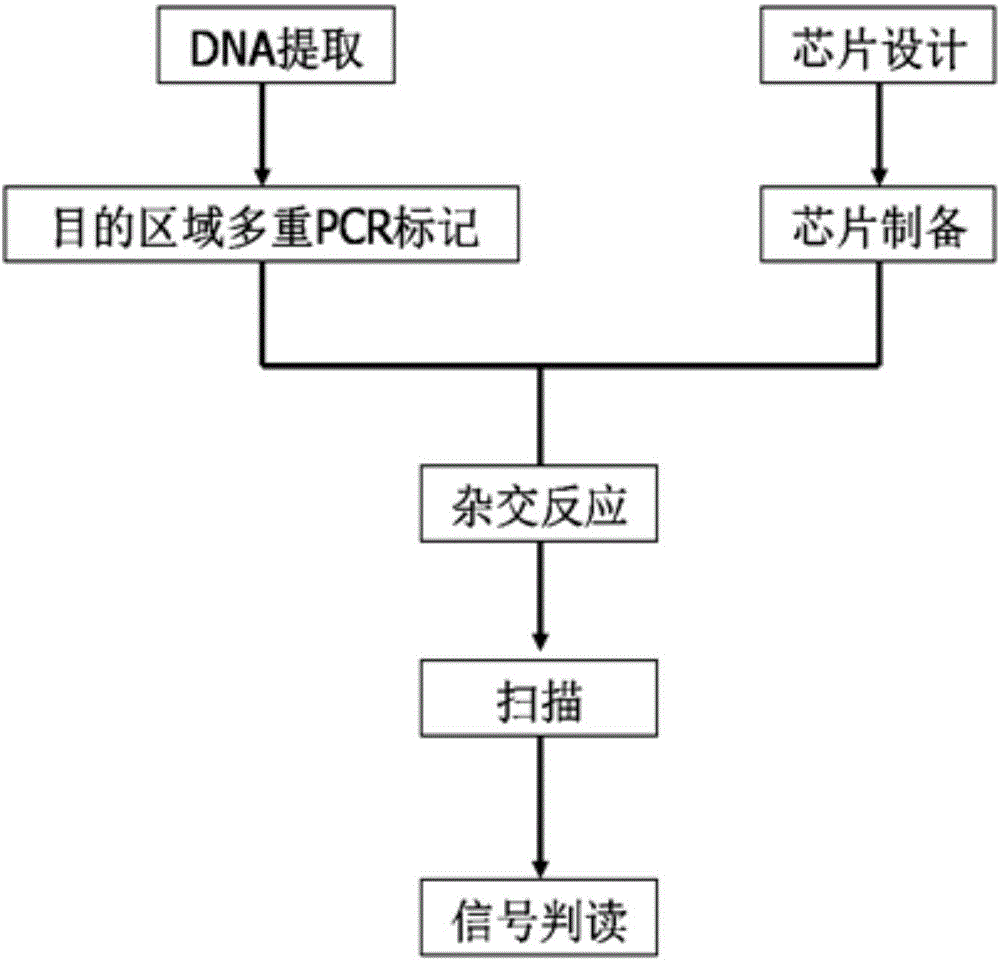
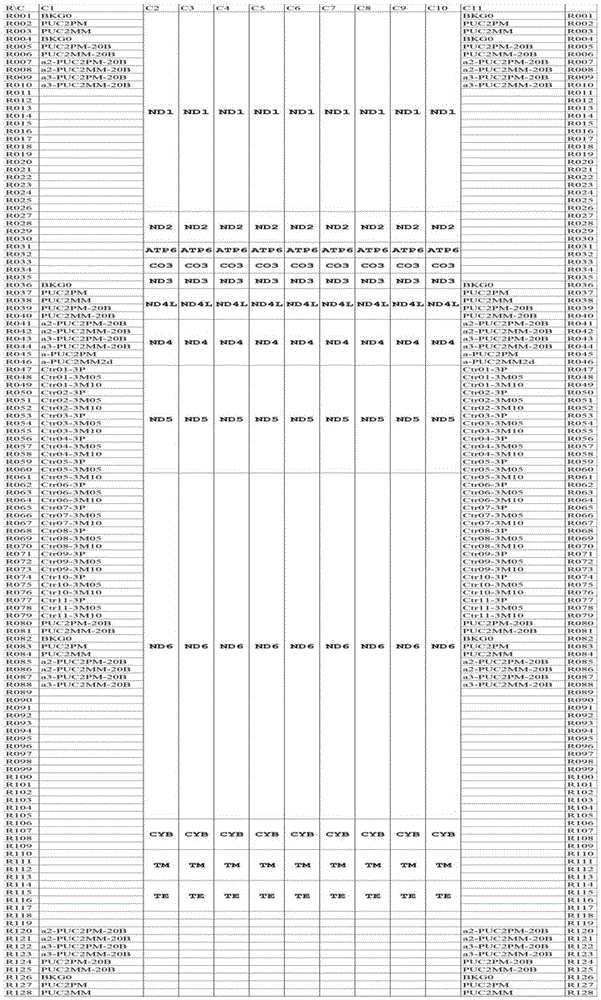
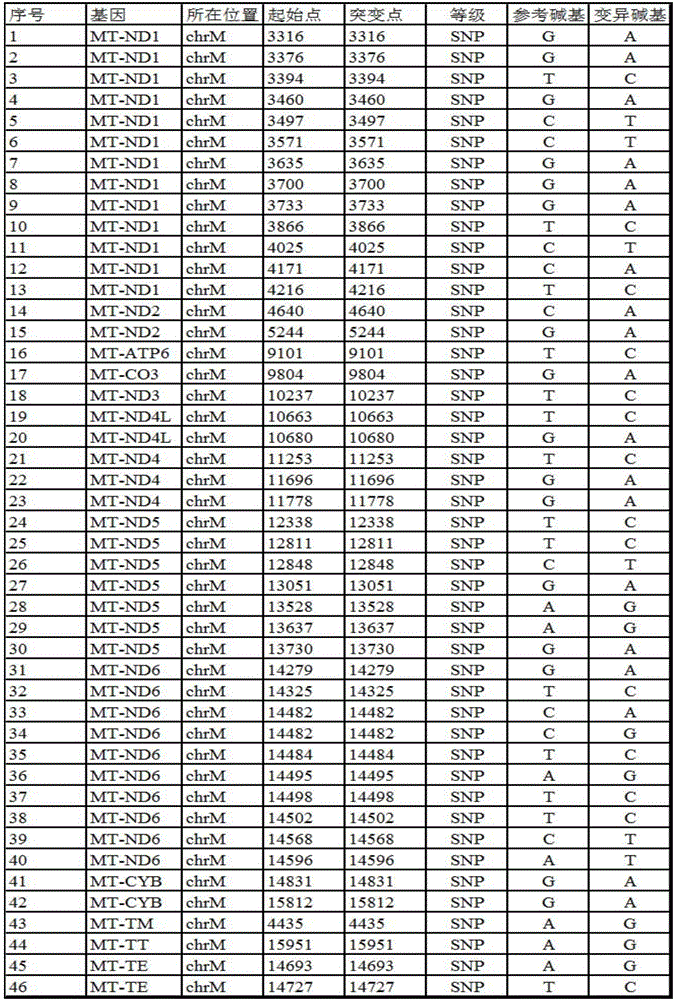
The invention provides a gene detection method of Leber's hereditary optic neuropathy, (LHON), a gene chip and a kit. The kit comprises a plurality of PCR probes and primers for detecting SNP of optic nerve lesion genes. The chip is provided with detection probes aiming at 13 mitochondrial genes and 46 SNP loci. According to the gene detection method, the kit and the gene chip are utilized to perform detection, the gene detection method comprises the following steps: performing amplification and hybridization on marked samples; scanning hybridization signals; obtaining chip data, and processing the obtained chip data; judging SNP loci, and judging and reading SNP loci information of the visual lesion genes. The gene detection method disclosed by the invention overcomes the detects that by adopting the direct sequencing, the using sample volume is large and the blood sample quantity is large, the amplification time is long, the amplification frequency is high, and false positive or false negative results can be easily caused, and the like, so that the pain of detected persons can be relieved, and not only be the detection accuracy improved, but also the detection time is shortened. According to the invention, the chip and the kit which have the characteristics of being rapid and being high in flux are provided, so that the early diagnosis and treatment of diseases can be facilitated, and nationwide large-scale screening and preventive inspection are convenient to carry out.
Owner:ZHEJIANG UNIV
Leber's hereditary optic neuropathy gene diagnosis kit or reagent
ActiveCN104046687AFully closedSensitivity is not goodMicrobiological testing/measurementWild typeMitophagy
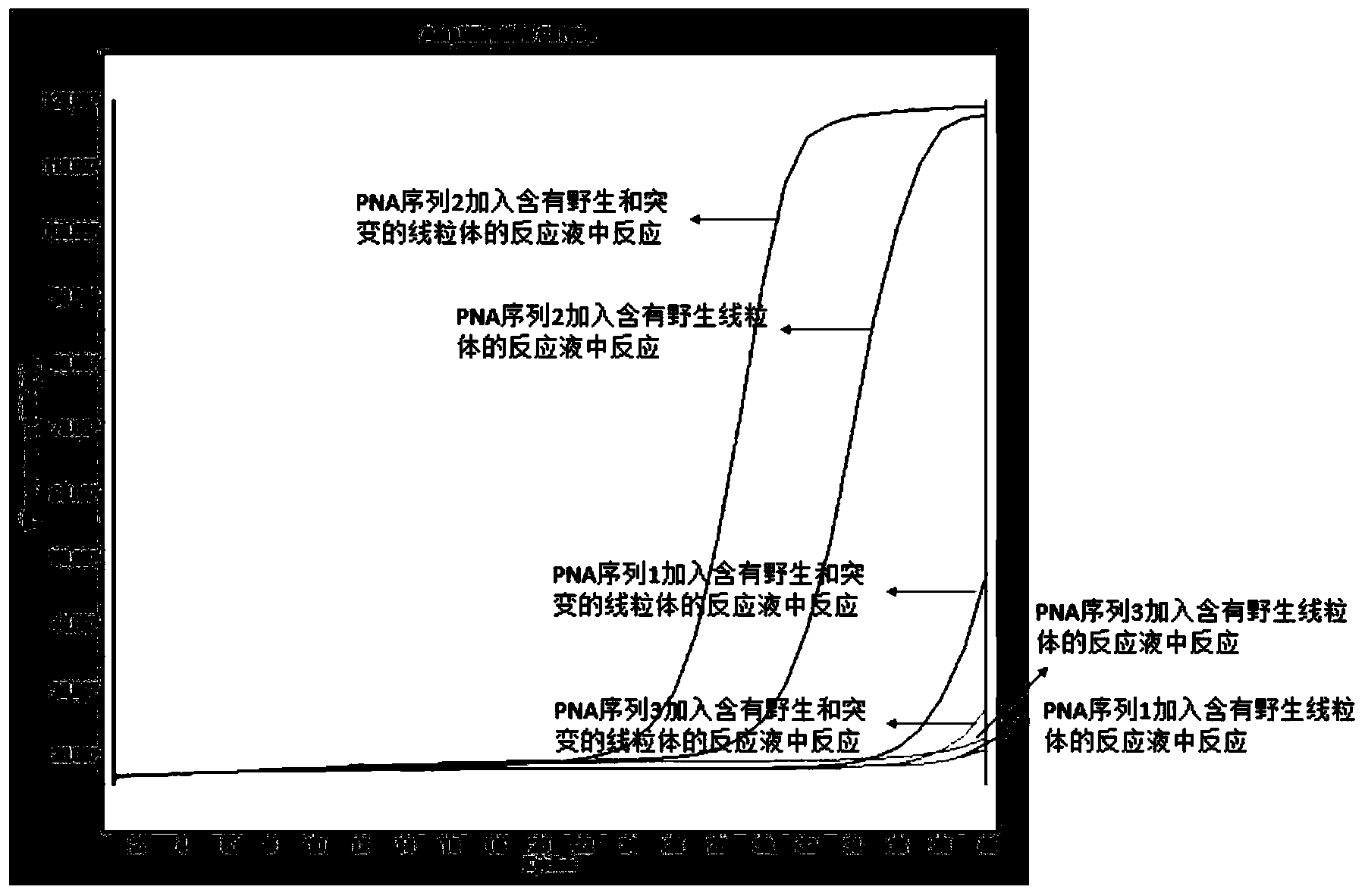
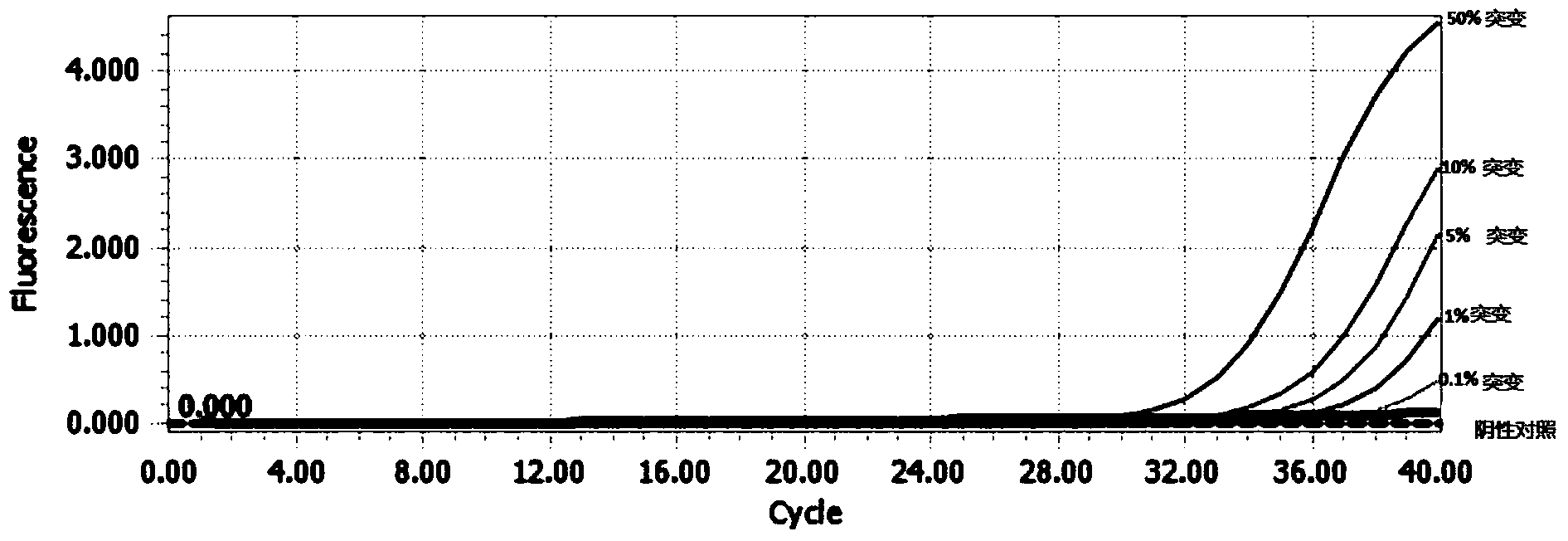
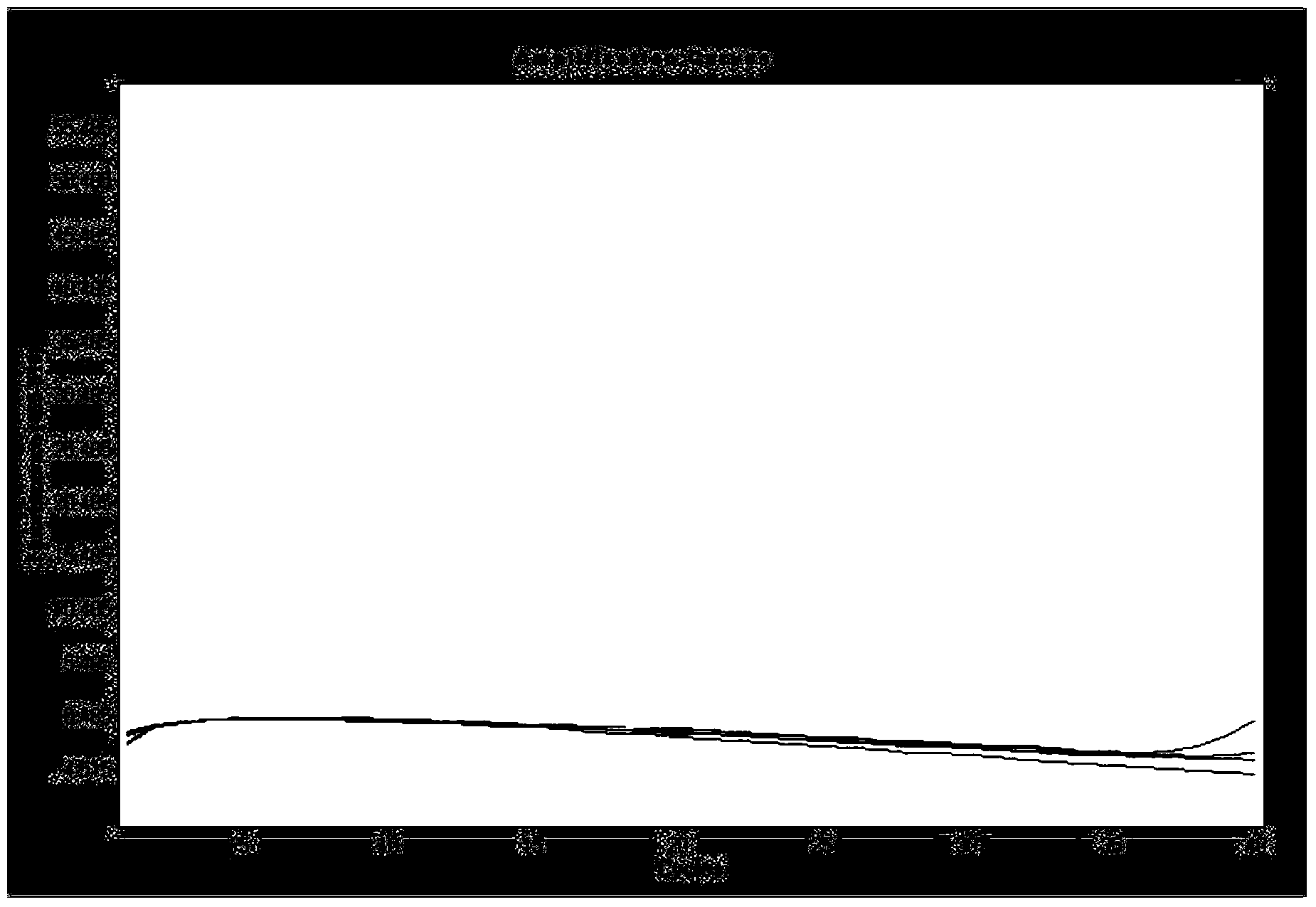
The invention relates to the field of medicine, and particularly relates to a Leber's hereditary optic neuropathy (LHON) gene diagnosis kit or reagent comprising a PCR primer mixture liquid; the primer mixture liquid comprises a pentose nucleic acid (PNA) probe for sealing a wild-type mitochondrion (which is a normal human mitochondrion without generation of mutation) deoxyribonucleic acid (DNA) and forward and reverse primers for amplifying mutant-type mitochondrion DNA. The kit or reagent has the advantages of simple operation, high sensitivity and good specificity, and is an efficient, sensitive, stable and specific LHON gene diagnosis detection technology.
Owner:同昕生物技术(北京)有限公司
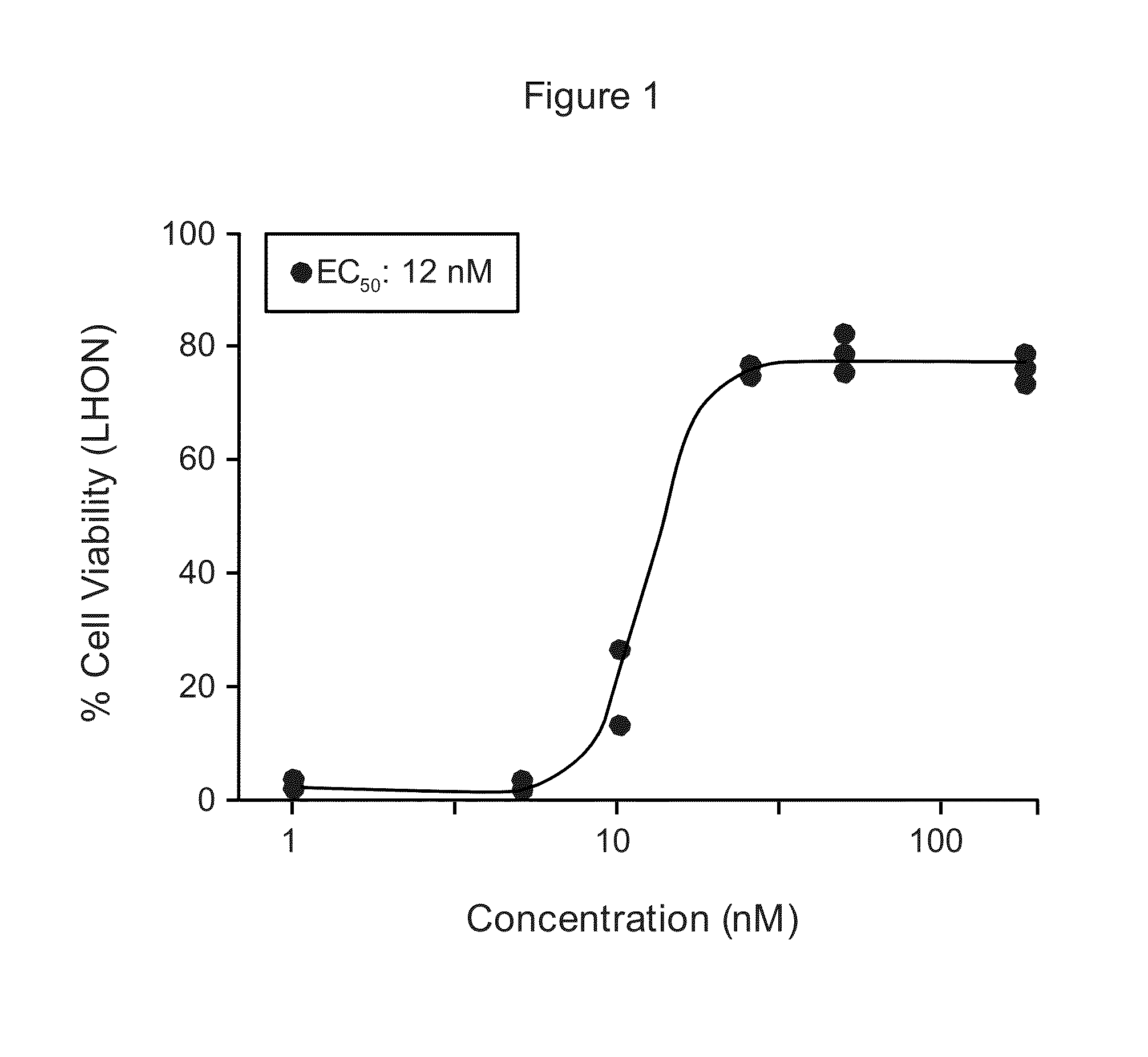
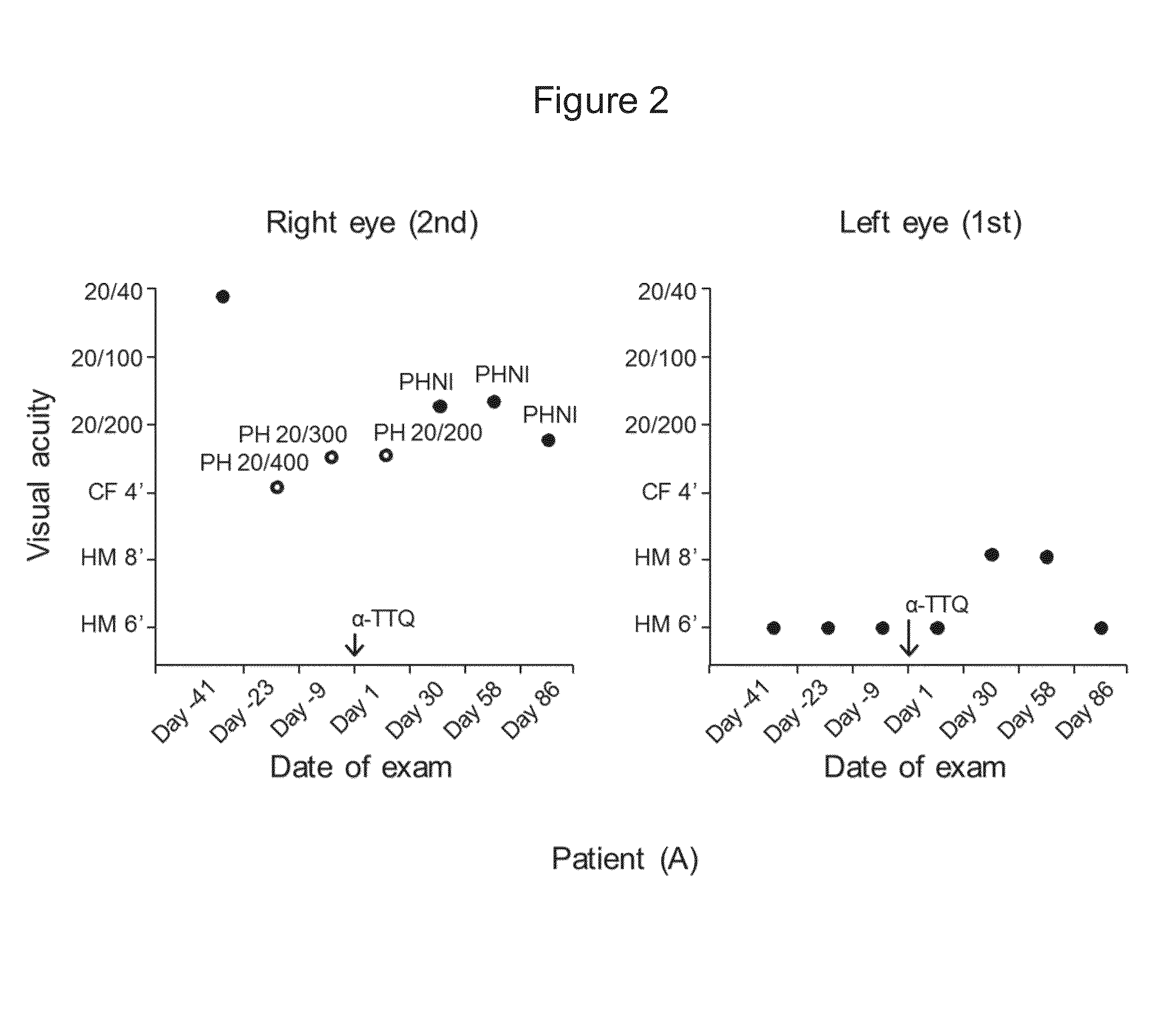
Compositions and methods for treating leber?s hereditary optic neuropathy
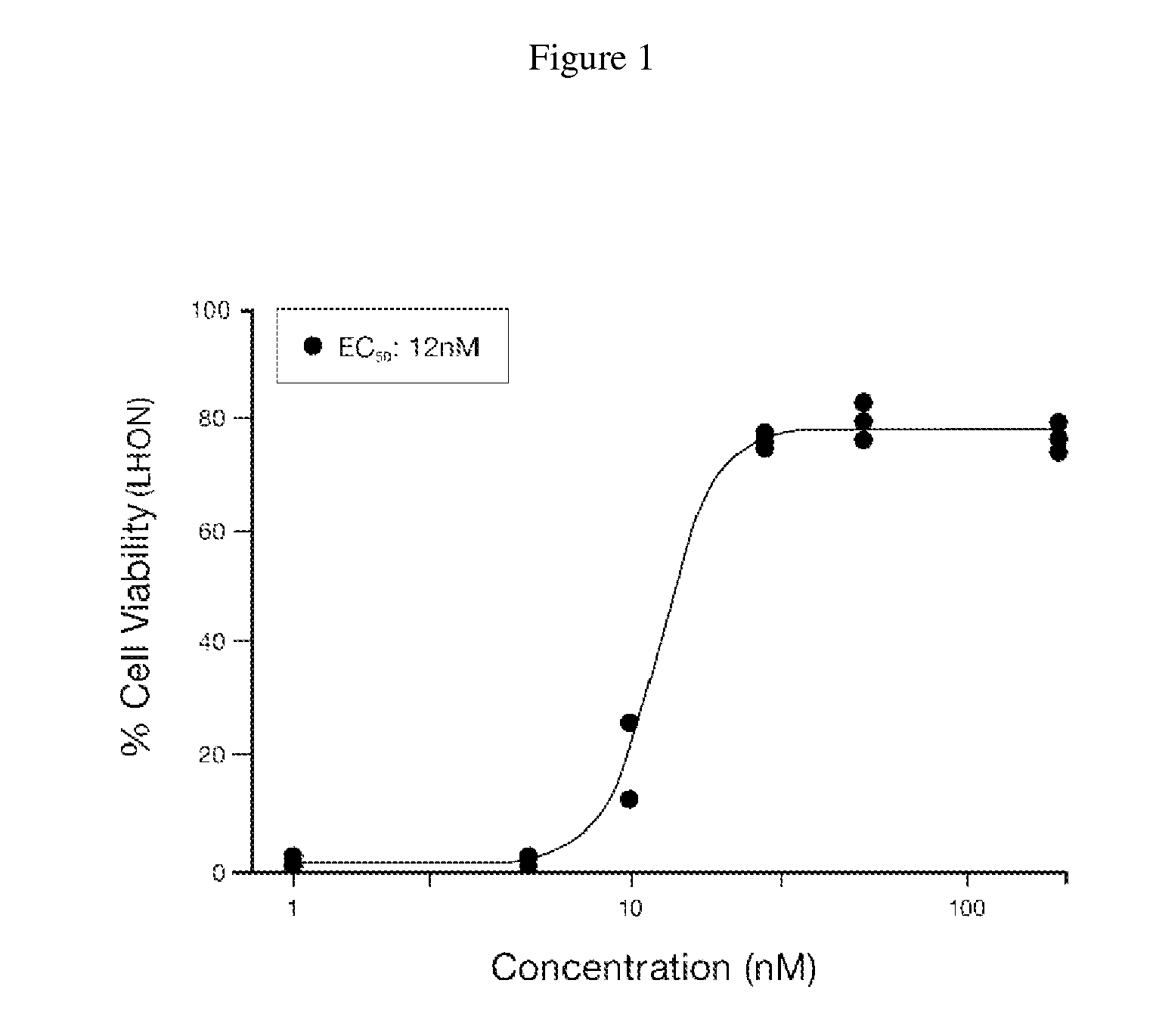
ActiveUS20200263172A1High average recovery of visionOrganic active ingredientsSenses disorderInherited optic neuropathyPharmaceutical drug
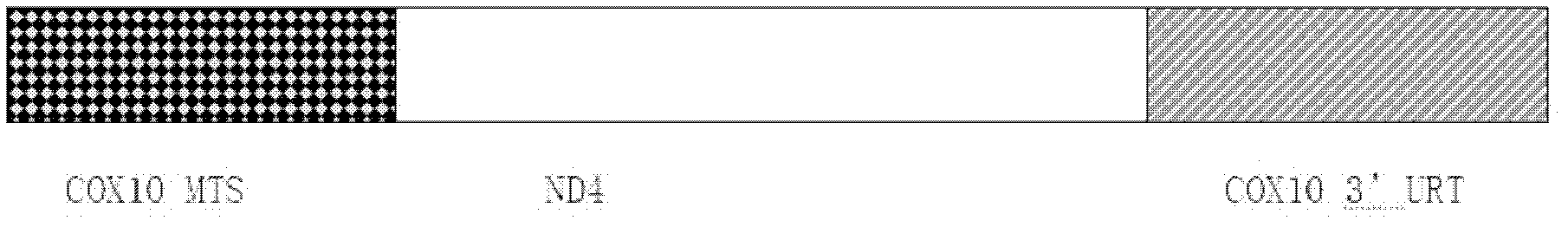
Disclosed herein is a recombinant nucleic acid, comprising: a mitochondrial targeting sequence; a mitochondrial protein coding sequence, wherein said mitochondrial protein coding sequence encodes a polypeptide comprising a mitochondrial protein; and a 3′UTR nucleic acid sequence. Also disclosed is a pharmaceutical composition comprising the recombinant nucleic acid and a method of treating Leber's hereditary optic neuropathy (LHON) using the pharmaceutical composition.
Owner:WUHAN NEUROPHTH BIOTECHNOLOGY LTD CO
Regulation of receptor expression through delivery of artificial transcription factors
InactiveCN103998609APolypeptide with localisation/targeting motifSenses disorderPromoterHigh affinity receptor
The invention relates to an artificial transcription factor comprising a polydactyl zinc finger protein targeting specifically a receptor gene promoter fused to an inhibitory or activatory protein domain, a nuclear localization sequence, and a protein transduction domain. In particular examples these receptor gene promoters regulate the expression of the endothelin receptor A, the endothelin receptor B, the Toll-like receptor 4 or the high-affinity IgE receptor. Artificial transcription factors directed to the endothelin A or B receptors are useful in the treatment of diseases modulated by endothelin, such as cardiovascular diseases, and, in particular, eye diseases, e.g. retinal vein occlusion, retinal artery occlusion, macular edema, optic neuropathy, central serous chorioretinopathy, retinitis pigmentosa, Leber's hereditary optic neuropathy, and the like. Artificial transcription factors directed to the Toll-like receptor 4 or the IgE receptor are useful for the treatment of autoimmune disorders, and the like, and allergic disorders, respectively.
Owner:ALIOPHTHA
Compositions and methods for treating leber's hereditary optic neuropathy
ActiveCN110876269AOrganic active ingredientsSenses disorderPharmaceutical drugNucleic acid sequencing
Provided is a recombinant nucleic acid, comprising: a mitochondrial targeting sequence; a mitochondrial protein coding sequence, wherein said mitochondrial protein coding sequence encodes a polypeptide comprising a mitochondrial protein; and a 3'UTR nucleic acid sequence. Also provided are a pharmaceutical composition comprising the recombinant nucleic acid and a method of treating Leber's hereditary optic neuropathy (LHON) using the pharmaceutical composition.
Owner:WUHAN NEUROPHTH BIOTECHNOLOGY LTD CO
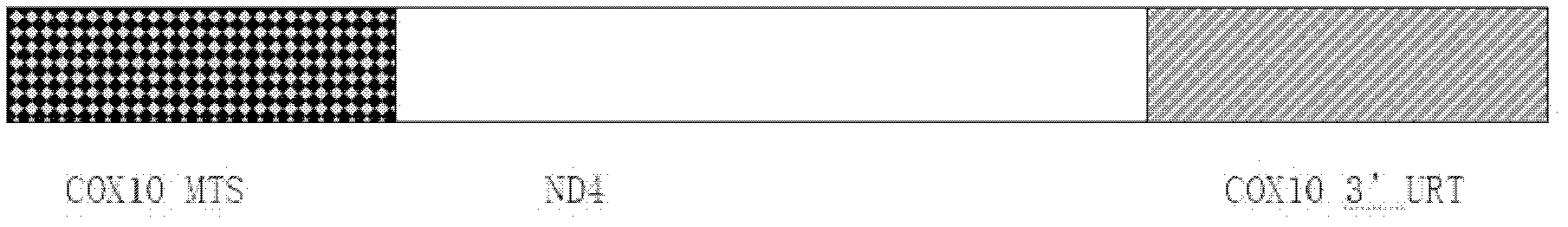
Recombinant adeno-associated virus-nadh dehydrogenase subunit 4 gene full-length and agent for the treatment of leber hereditary optic neuropathy
ActiveCN104450747BEfficient transfectionHigh expressionSenses disorderNervous disorderNucleotideMitophagy
The invention discloses a recombinant adeno-associated virus-NADH dehydrogenase subunit 4 gene full length and a medicament for treating Leber hereditary optic neuropathy. The full length of the gene is shown in the nucleotide sequence of SEQ ID NO: 1. Wherein, the nucleotide sequence has a full length of 3824bp, which consists of a CAG promoter sequence, a coding sequence of ND4 with a Cox10 mitochondrial localization sequence and a UTR with a length of 625bp. The medicament is injected into the vitreous cavity of the eye for the treatment of Leber's hereditary optic neuropathy. The medicament of the present invention is injected into the vitreous cavity, can maintain vitality in the vitreous cavity, and can be efficiently transfected into optic nerve cells. The signal peptide at the front end of the protein N, Orientation guides the protein into the mitochondria, and the mature ND4 protein enters the mitochondria to play a role. Therefore, drugs are effective in the treatment of Leber hereditary optic neuropathy.
Owner:WUHAN NEUROPHTH BIOTECHNOLOGY LTD CO
Leber hereditary optic neuropathy gene drug
ActiveCN109207520ASimple injectionEfficient transductionSenses disorderPeptide/protein ingredientsLEBER HEREDITARY OPTIC NEUROPATHYIn vivo experiment
The invention provides a recombinant adeno-associated virus-mediated Leber hereditary optic neuropathy therapeutic agent gene drug. The recombinant adeno-associated virus vector carries the nuclear expression cassette of ND4 protein. In vivo experiments show that the recombinant adeno-associated virus vector could be efficiently introduced into the retina by intravitreal injection, and the ND4 protein is continuously and stably expressed and localized in mitochondria to alleviate or recover the adverse symptoms caused by mutation of ND4 gene. The results suggest that the recombinant adeno-associated virus vector might be a promising drug for the treatment of Leber hereditary optic neuropathy.
Owner:BEIJING GENECRADLE PHARM CO LTD
Compositions and methods for treating leber's hereditary optic neuropathy
Provided is a recombinant nucleic acid, comprising: a mitochondrial targeting sequence; a mitochondrial protein coding sequence, wherein the mitochondrial protein coding sequence encodes a polypeptidecomprising a mitochondrial protein; and a 3'UTR nucleic acid sequence. Also provided are a pharmaceutical composition comprising the recombinant nucleic acid and a method of treating Leber's hereditary optic neuropathy (LHON) using the pharmaceutical composition.
Owner:WUHAN NEUROPHTH BIOTECHNOLOGY LTD CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com