Leber hereditary optic neuropathy gene drug
A genetic drug and gene technology, applied in the direction of genetic material components, the use of vectors to introduce foreign genetic material, gene therapy, etc., can solve the problem of low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Plasmid vector construction
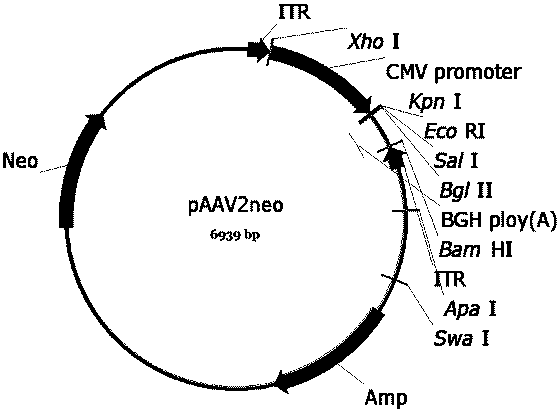
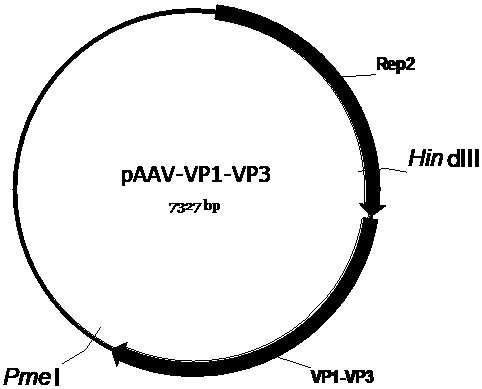
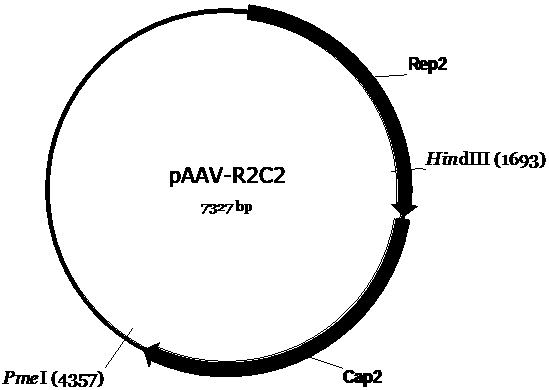
[0053] First, we construct the AAV vector plasmid (pAAV-CMV-EGFP-COX10 ( image 3 ), pscAVV-CAM-EGFP-ATP5B ( Figure 4 ), pAAV-CMV-ND4-COX10 ( Figure 5 ), pAAV-CMV-ocND4-COX10 ( Figure 6 ), pscAAV-CAM-ocND4-ATP5B ( Figure 7 ), pscAAV-HSP-mND4 ( Figure 8 )), and then constructed the mitochondria-targeted AAV vector packaging envelope plasmid (pAAV-VP2A-COX8-EGFP ( Figure 9 ) and pAAV-VP1-VP3 ( Figure 10 )) and the AAV vector packaging envelope plasmid (pAAV-R2C2-3YF( Figure 12 ), pAAV-R2C2-4YF ( Figure 13 )). The single-chain AAV vector packaging vector plasmid (pAAV-CMV-EGFP-COX10 ( image 3 ), pAAV-CMV-ND4-COX10 ( Figure 5 ), pAAV-CMV-ocND4-COX10 ( Figure 6 )) pAAV2neo held in company ( figure 1 ) is the basic skeleton. Double-stranded AAV vector packaging plasmid (pscAVV-CAM-EGFP-ATP5B ( Figure 4 ), pscAAV-CAM-ocND4-ATP5B ( Figure 7 ), pscAAV-HSP-mND4 ( Figure 8 )) is in the pscAAV-CAM vector ( fi...
Embodiment 2
[0069] Example 2 Preparation and assay of recombinant AAV virus
[0070] References (Xiao X, et al . J Virol. 1998;72(3):2224-2232.), the three-plasmid packaging system was used to package the recombinant AAV virus, and the cesium chloride density gradient centrifugation method was used to separate, purify and package the AAV virus. Briefly, AAV vector plasmid (pAAV-CMV-EGFP-COX10, pscAAV-CAM-EGFP-ATP5B, pAAV-CMV-ND4-COX10, pAAV-CMV-ocND4-COX10, or pscAAV-CAM-ocND4-ATP5B), helper plasmid (pHelper) and AAV Rep and Cap protein expression plasmids (pAAV-DJ (for packaging AAVDJ virus), pAAV-R2C2, pAAV-R2C2-3YF or pAAV-R2C2-4YF) were mixed at a molar ratio of 1:1:1 After homogenization, HEK293 cells were transfected by the calcium phosphate method. After 48 hours of transfection, the cells and culture supernatant were harvested, and the recombinant AAV virus was isolated and purified by cesium chloride density gradient centrifugation. Packaged and purified to obtain AAVDJ-EGFP-CO...
Embodiment 3
[0094] Example 3 Carrying EGFP reporter gene AAV virus in vitro cell experiment
[0095] Using the three-plasmid packaging system, the pAAV-CMV-EGFP-COX10 and pscAAV-CAM-EGFP-ATP5B plasmids were packaged into AAVDJ-EGFP-COX10 and scAAVDJ-EGFP-ATP5B, respectively. The packaged AAVDJ-EGFP-COX10 and scAAVDJ-EGFP-ATP5B viruses were divided into 1×10 5 A dose of vg / cell was used to infect human BJ cells (purchased from ATCC (American Tissue Culture Collection, American Tissue Culture Collection), ATCC NO.CRL-2522). After the virus transduced the cells for 72 hours, the expression of green fluorescent protein and its distribution in the cells were observed with a confocal microscope (Leica TSC 4D). Localization of mitochondria in cells with the alpha subunit of ATP synthase. Under the same field of view, the overlapping area between the distribution of GFP in cells and the distribution of ATP synthase α subunit is the distribution area of GFP in mitochondria. For the detection ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com