Series of polymers with acid-sensitive degradation and temperature-sensitive properties and drug-loading composition thereof
A polymer and property technology, applied in the fields of biomedicine and polymer medical materials, can solve the problems of chemical cross-linking monomer toxicity, high water content increases the risk of deterioration, unstable drug release properties, etc., and achieves good drug release properties , controllable degradation, and convenient administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
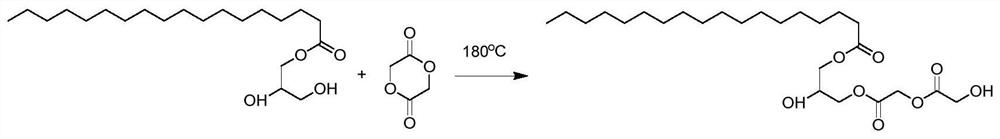
[0111] Embodiment 1 A kind of preparation method of oligomer monomer
[0112] The oligomer monomers of this example were prepared from glyceryl monostearate (GMS) and glycolide (GA). The molar ratio of the two components is 1:1.
[0113] as attached figure 1 As shown, weigh glyceryl monostearate (GMS) (17.928g, 0.05mol) and glycolide (GA) (5.8035g, 0.05mol) in a pressure-resistant reaction tube, under the protection of an inert gas, at 180 ℃ airtight heating and stirring for 24h, the product obtained is glyceryl monostearate diglycolide (GMS-diGL).
Embodiment 2
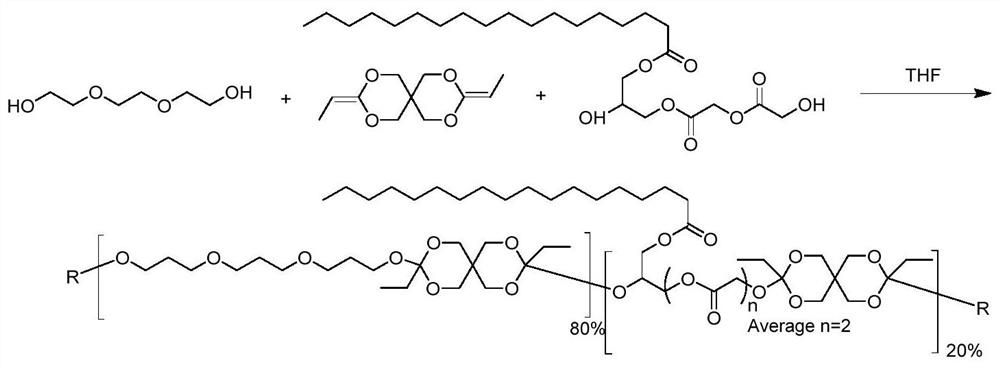
[0114] Example 2 A method for preparing a polymer with acid-sensitive degradation and positive temperature-sensitive properties
[0115] The polymer with acid-sensitive degradation and positive temperature-sensitive properties in this example was prepared from 3,9-bis(ethylene)-2,4,8,10-tetraoxaspiro[5,5]undecane (DETOSU), triethylene glycol (TEG) and glyceryl monostearate diglycolide (GMS-diGL). The molar ratio of the three components (DETOSU:TEG:GMS-diGL) was 90:80:20.
[0116] as attached figure 2 As shown, under strict anhydrous conditions, DETOSU (1.910 g, 0.009 mol) was dissolved in 15 ml of anhydrous tetrahydrofuran (THF) and TEG (1.2014 g, 0.008 mol) in a 50 ml flask, and GMS-diGL (0.9493 g , 0.002mol) was dissolved in 5ml of anhydrous THF. The GMS-diGL solution was added to the solution of DETOSU and TEG to initiate polymerization. Within minutes, the solution reached boiling point. The solution was allowed to cool to room temperature, then concentrated by rotar...
Embodiment 3
[0117] Example 3 A method for preparing a polymer with acid-sensitive degradation and positive temperature-sensitive properties
[0118] The polymer with acid-sensitive degradation and positive temperature-sensitive properties in this example was prepared from 3,9-bis(ethylene)-2,4,8,10-tetraoxaspiro[5,5]undecane (DETOSU), triethylene glycol (TEG) and glyceryl monolaurate diglycolide (GML-diGL). The molar ratio of the three components (DETOSU:TEG:GML-diGL) was 95:80:20.
[0119] Under strict anhydrous conditions, DETOSU (2.0164 g, 0.0095 mol) was dissolved in 15 ml of anhydrous (tetrahydrofuran) THF and TEG (1.2014 g, 0.008 mol) in a 50 ml flask, and GML-diGL (0.7807 g, 0.002 mol ) was dissolved in 5 ml dry THF. The GML-diGL solution was added to the solution of DETOSU and TEG to initiate polymerization. Within minutes, the solution reached boiling point. The solution was allowed to cool to room temperature, then concentrated by rotary evaporation at 50°C, followed by rota...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com