Compositions and methods for treating leber's hereditary optic neuropathy
A variant and recombinant nucleic acid technology, which is applied in the fields of genetic material components, nervous system diseases, introduction of foreign genetic material using vectors, etc., can solve problems such as the reduction of mitochondrial NADH dehydrogenase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0213] Preparation of vector
[0214] The vectors of the present application can be prepared by standard methods known in the art to provide vectors for gene therapy. Thus, well-established published transfection, assembly and purification methods can be used to prepare suitable vectors.
[0215] As mentioned above, in addition to the polynucleotide sequence encoding ND4, ND6 or ND1 or variants thereof, the vector of the present application may also contain the entire genome of a naturally occurring AAV virus. Typically, however, a derivatized genome will be used, eg having at least one inverted terminal repeat (ITR), but which may lack any AAV genes, eg rep or cap.
[0216] In such embodiments, in order for the derived genome to be packaged into an AAV virion, other genetic constructs providing AAV and / or helper virus function will be combined with the derived genome in the host cell. These additional constructs typically contain genes encoding AAV capsid proteins, cap, VP1...
Embodiment 1
[0289] Embodiment 1-ND4 plasmid and virus preparation
[0290] 1.1 Plasmid preparation
[0291] The nucleotide sequence (SEQ ID NO: 6) for human ND4 was obtained based on the National Center for Biotechnology Information reference sequence yp_003024035.1. The sequence of the unoptimized mitochondrial targeting sequence COX10 is SEQ ID NO:1. Optimized sequences for the mitochondrial targeting sequence COX10 (opt_COX10, SEQ ID NO:2) and the coding sequence of human ND4 (opt_ND4, SEQ ID NO:7) were designed to improve transcription efficiency and translation efficiency. The optimized COX10-ND4 sequence has approximately 75.89% homology to unoptimized COX10-ND4, followed by the untranslated region (i.e., 3' UTR, SEQ ID NO: 13), forming the recombinant nucleic acid, opt_COX10-opt_ND4-3' UTR (shown in SEQ ID NO: 31).
[0292] The synthetic recombinant nucleic acid opt_COX10-opt_ND4-3'UTR was incorporated into the adeno-associated virus (AAV) vector by PCR amplification ( figure 1...
Embodiment 2
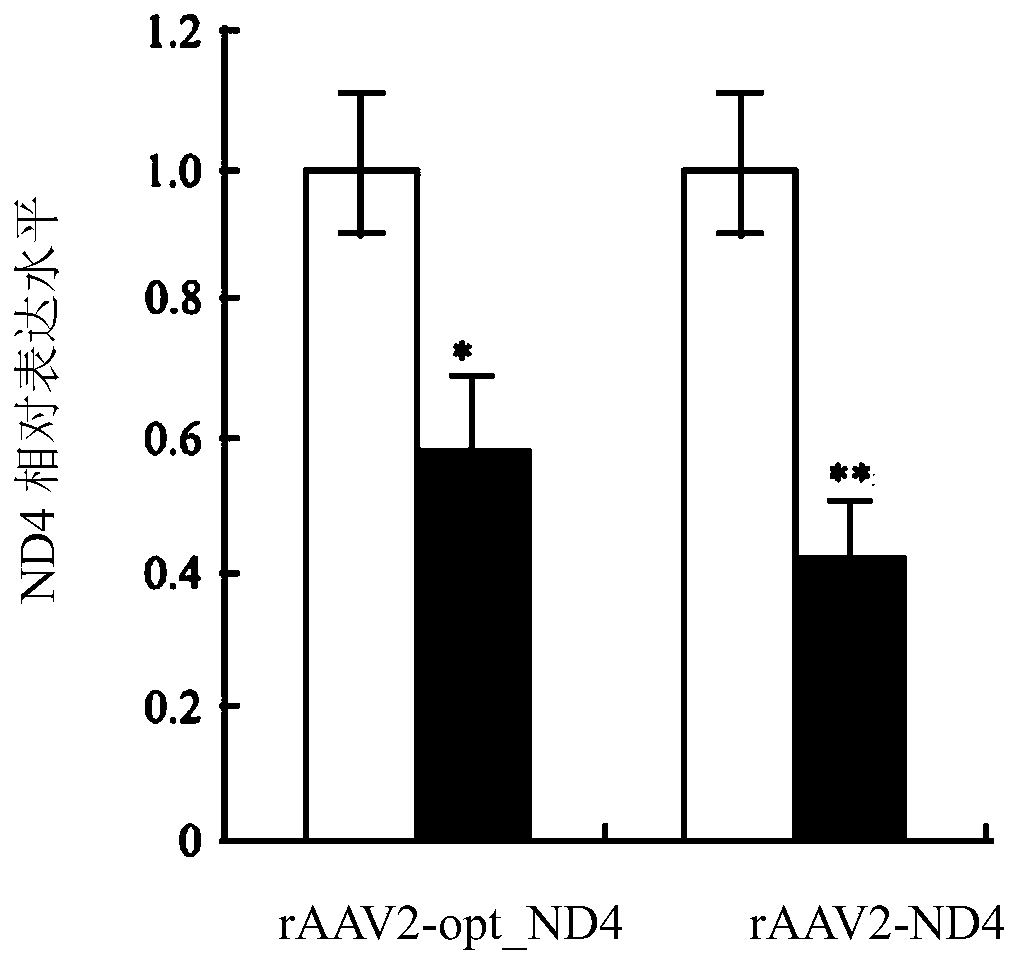
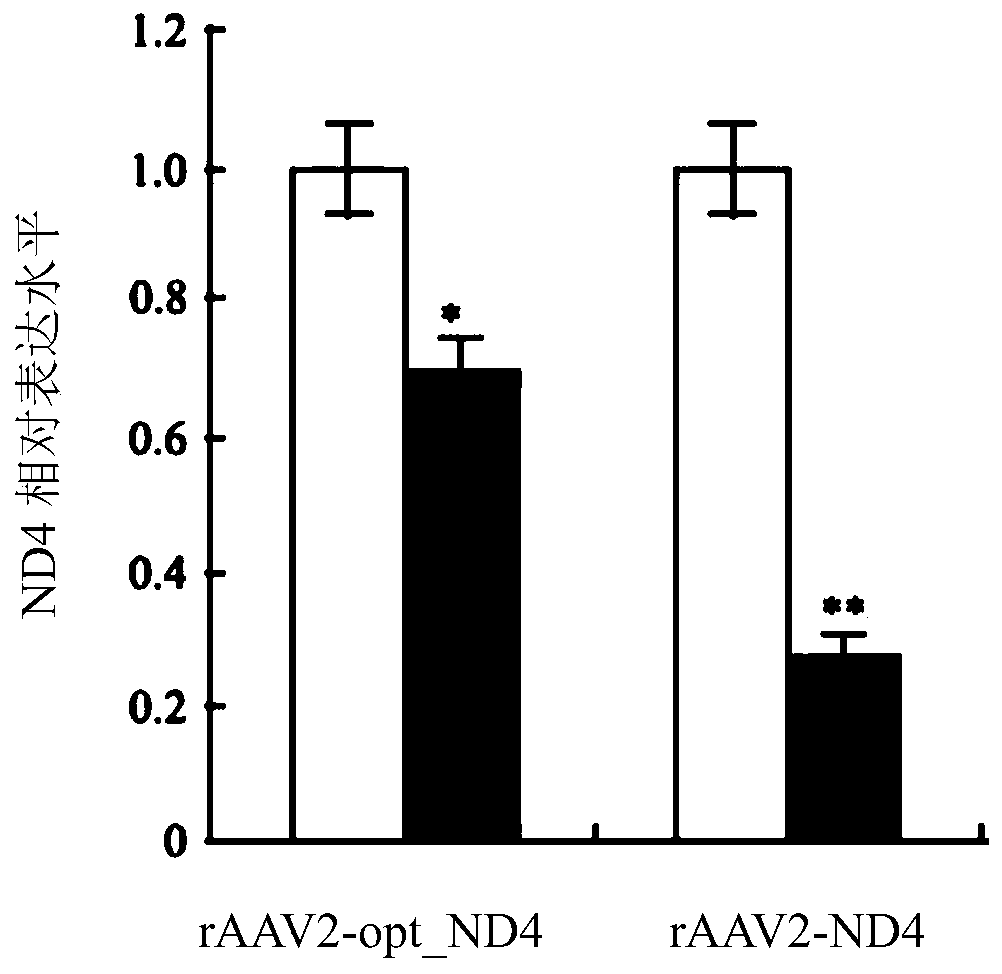
[0302] Example 2 - Intravitreal injection of rAAV2 in rabbit eyes
[0303] Twelve rabbits were divided into two groups: rAAV2-ND4 and rAAV2-optimized ND4. Virus solution (1×10 10 vg / 0.05mL) into the vitreous from the vitreous at 3 mm lateral to the limbus. After intravitreal injection, examine the eye using a slit lamp and fundus photography. Inject for 30 days. Each group was tested by RT-PCR and immunoblotting.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com