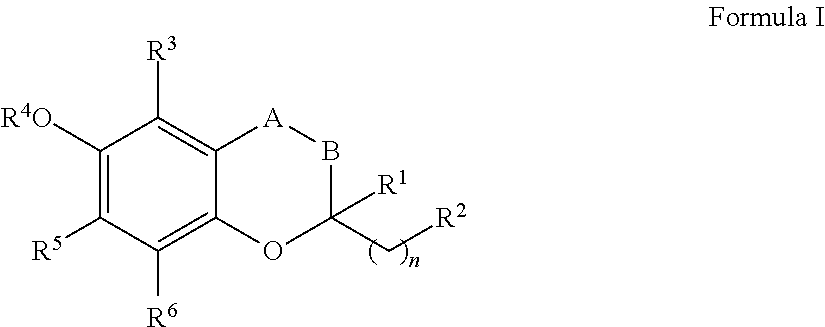
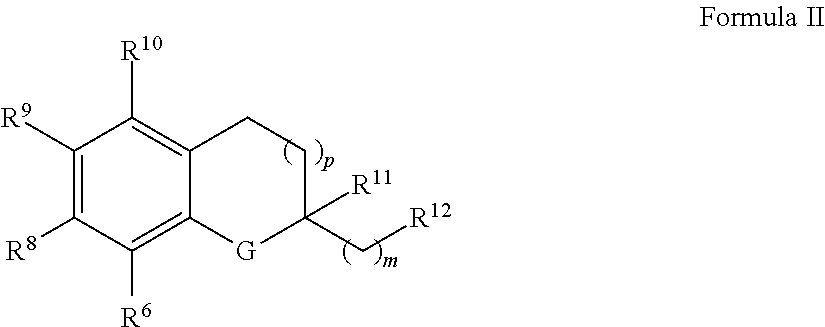
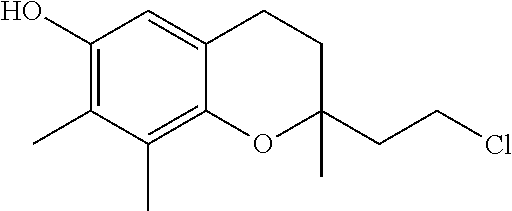
Treatment of mitochondrial diseases
a technology for mitochondrial diseases and treatment methods, applied in the field of treatment, amelioration, or prevention of mitochondrial diseases, can solve the problems of difficulty in walking, loss of muscle control and strength in hands and arms, and difficulty in breathing, so as to limit or prevent damage to organelles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Media Composition
[0193]Neurobasal / B27i: Neurobasal medium plus 1×B27 supplement, 0.5 mM L-glutamine, 25 μM L-glutamic acid, and 0.5× Penicillin / Streptomycin
Neurobasal / B27m: Neurobasal medium plus 1×B27 supplement and 0.5 mM L-glutamine
BSS (Ca / Mg free): HBSS (calcium / magnesium free) plus 10 mM Hepes (pH 7.25), 1×Penicillin / Streptomycin, and 1 mM Sodium Pyruvate
Glucose-free BSSo: 143.6 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, 1 mM NaH2PO4, 26.2 mM NaHCO3, 10 mg / l phenol red, 0.25× Penicillin / Streptomycin, and 10 mM Hepes (pH 7.4)
Papain Quench solution: Neurobasal medium plus 1×B27 supplement, 1× Penicillin / Streptomycin and 0.5 mg / ml DNase1
Assay media: Neurobasal medium plus 1×B27 (minus AO) supplement, 0.5 mM L-glutamine, and 0.25× Penicillin / Streptomycin.
Experimental Procedure
Hippocampal Cell Culture
[0194]Hippocampal neurons were isolated from E18 rat embryos as follows. Embryos were decapitated and the heads immersed in cold BSS (Ca / Mg free). Usi...
example 2
Media Composition
[0201]RF media: DMEM-No glucose, glucose (29.1 mM), L-glutamine (1.4 mM), 10% heat-inactivated FBS, and 1× penicillin / streptomycin (P / S)
Wash media: DMEM-No glucose and 1×P / S
Low serum media: DMEM-No glucose, glucose (29.1 mM), L-glutamine (1.4 mM), 0.5% FBS, and 1×P / S
Assay Media: DMEM-No glucose, L-glutamine (1.4 mM), 0.5% FBS, and 1×P / S
Experimental Procedure
[0202]A substantia nigra-derived dopaminergic progenitor cell line was seeded in poly-D-lysine-coated 24-well plates at a density of 4500 cells per well in RF media. The cells were left to attach for 16 hours in a 33° C. incubator (5% CO2) after which time they were washed once with 500 μL wash media and then differentiated into a neuronal phenotype by incubating in low serum media for 24 hours in a 39° C. incubator (5% CO2).
[0203]After 24 hours the low serum medium was aspirated from the cells and the monolayer was washed once with 500 μL wash media. Test articles were diluted to 2-fold the ...
example 3
[0216]Male C57 / BL6 mice (Harlan, Ind.), weight 25-30 g, were used in all studies. MPTP-HCl (Sigma) was administered i.p. according to one of the following protocols. The maximum volume which was given per injection is 200 μL. In all studies, animals were euthanized with carbon dioxide and, brains were removed for subsequent determination of dopamine depletion where appropriate.
Subacute Model
[0217]Animals received 25 mg / kg of MPTP once a day for 5 consecutive days. The end point was 2 days after the final dose.
Acute Model
[0218]Animals received 4×20 mg / kg of MPTP at 2 hour intervals. The end point was at either 7 or 14 days.
Subchronic Model
[0219]Animals received 2×40 mg / kg of MPTP with this repeated 16 hours later. The end point was at either 14 or 28 days.
Chronic Model
[0220]Animals received 25 mg / kg of MPTP, given twice weekly for 5 weeks. The end point was either 1, 3 or 24 weeks after the final dose.
Neurobehavioral Outcome Measures
[0221]Compound efficacy was examin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| diameters | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com