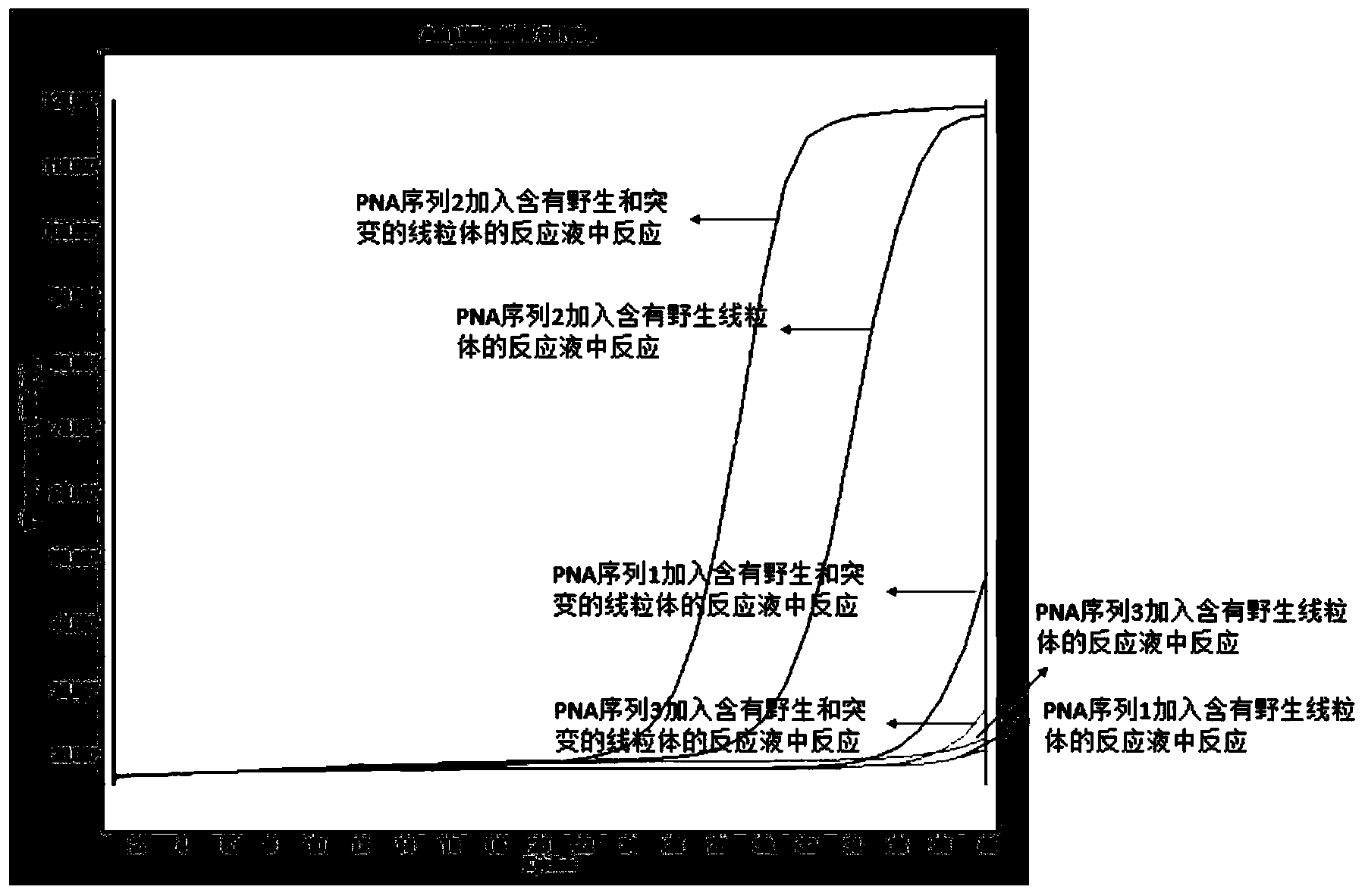
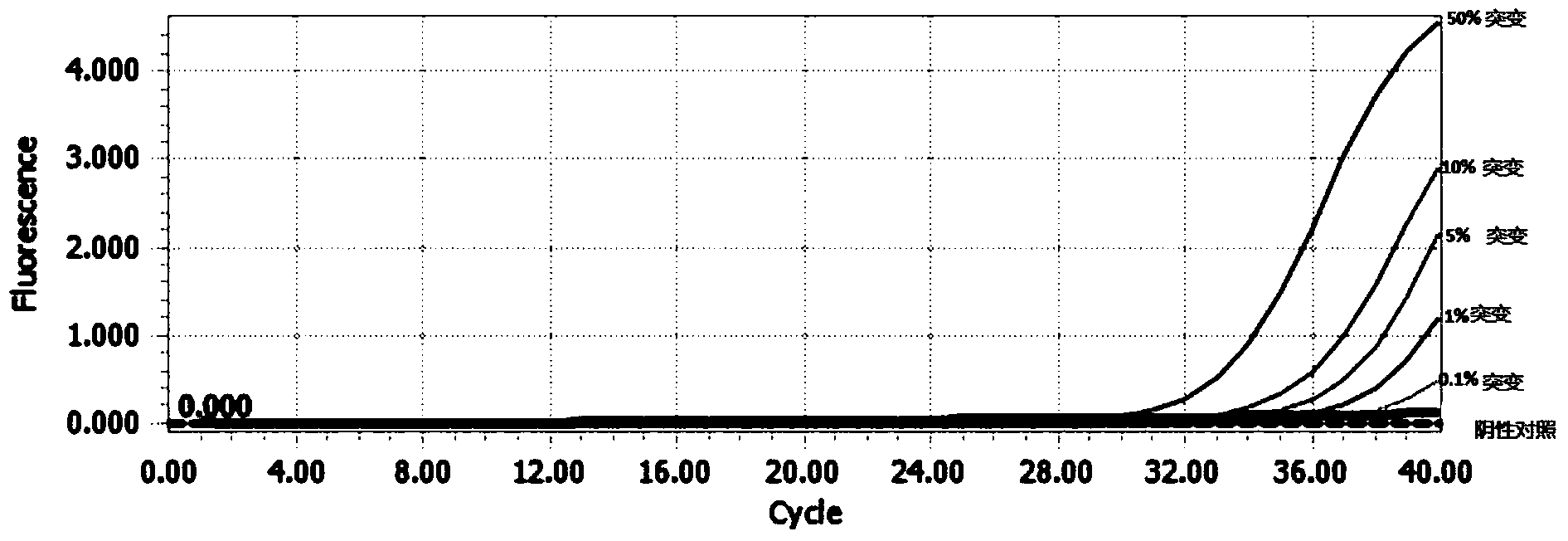
Leber's hereditary optic neuropathy gene diagnosis kit or reagent
A technology for optic neuropathy and gene diagnosis, applied in the field of medical reagents, can solve the problems of kits and reagents that have not yet had wild mitochondrial DNA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Leber hereditary optic neuropathy gene diagnosis kit of the present invention
[0066] Table 2 The composition of the kit (10 servings)
[0067] kit components
volume
effect
1. TransStart TM Green qPCR SuperMix(2X)
200ul
PCR reaction mix
2. Primer solutionⅠ
100ul
Primer mix to detect the 3460 mutation
3. Primer solutionⅡ
100ul
Primer mix to detect the 11778 mutation
4. Primer solutionⅢ
100ul
Primer mix to detect the 14484 mutation
5. negative control (1ng / ul)
50ul
6. Positive control (0.2ng / ul)
50ul
[0068] The DNA sequence of Primer solution I in Table 3 is:
[0069]
[0070] The sequence of Primer solution II in Table 4 is:
[0071]
[0072] The sequence of Primer solution III in Table 5 is:
[0073]
Embodiment 2
[0075] The kit of implementation 1 was taken in the following manner: 30 clinical blood samples were taken, and the sample DNA was first extracted using a DNA extraction kit, and the amount of DNA was adjusted to 1ng / ul. Negtive control and Positive control should be used as controls for each test.
[0076] 1) Configure the reaction system (20ul) in the following manner, mix well and place on Chromo4 quantitative PCR:
[0077] The way of adding samples is as follows:
[0078] Sample No. 1 needs 3 reaction tubes, and each tube detects a mutation point. For other samples, samples No. 2-30 are deduced by analogy.
[0079]
[0080]
[0081] Negative control reaction tube 3 tubes
[0082]
[0083] Positive control reaction tube 3 tubes
[0084]
[0085] 2) According to the following conditions for PCR reaction
[0086]
[0087] SyberGreen1 fluorescence was detected after the extension reaction.
[0088] 3 result judgment
[0089] 1) Threshold setting
[0090] C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com