In-vitro diagnostic kit of Leber hereditary optic neuropathy
A diagnostic kit and technology for optic neuropathy, which can be used in the determination/examination of microorganisms, biochemical equipment and methods, etc., can solve the problems of limited clinical use, complicated procedures, and high missed diagnosis rate, so as to reduce medical expenses and improve the quality of the population. , no false positive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
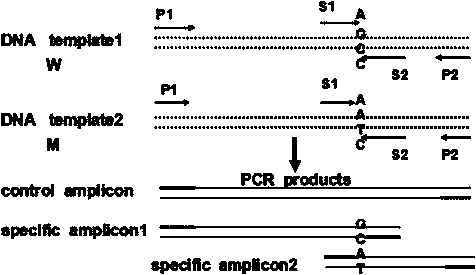
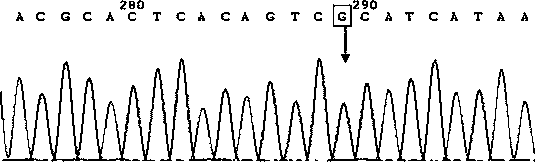
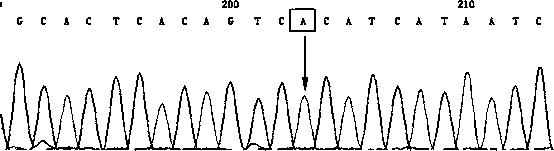
[0032] 1. Sample: DNA extracted from human whole blood (50-100ng / ul). Samples 1 and 2 are normal controls, which means that the amplification template is derived from normal human blood DNA without the following three mutations; samples 3 and 4 are homozygous mutants, which means that the amplification template is derived from the three sites positive reference plasmid DNA, the positive reference plasmid is m.11778G>A; m.14484T>C; m.3460G>A artificial mutation fragment plasmid; The amplification template is derived from the mixed template of positive reference plasmid and non-mutated normal human blood DNA.
[0033] 2. Reagents: Hot Start Green Master Mix, 2×: Contains 2×Green Go Taq Reaction Buffer (pH8.5), 400μM dATP, 400μM GATP, 400μM dCTP, 400μM dTTP, 4mM MgCl 2 , Nuclease-Free Water, DNA polymerase.
[0034] 3. T-ARMS-PCR reaction system and amplification procedure
[0035] ①Use the following primers, systems and procedures to perform PCR amplification on each sample...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com