Application of sorafenib to preparing medicine for preventing, alleviating and/or treating fatty liver and related diseases
A technology for fatty liver and steatohepatitis, which can be used in drug combinations, antineoplastic drugs, active ingredients of heterocyclic compounds, etc., can solve the problems such as the unreported treatment effect of NAFLD, save time and financial resources, have obvious curative effect, and reduce lipids. The effect of deposition inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
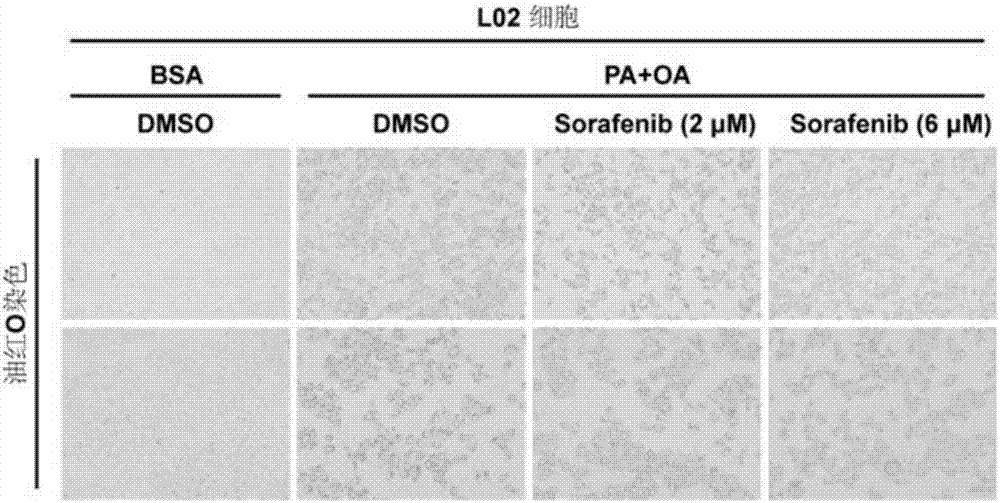
[0064] [Example 1] Effect of Sorafenib Pretreatment on Fat Accumulation in L02 Cells
[0065] The L02 cells were divided into 4 groups, namely DMSO BSA group, DMSO PA / OA group, sorafenib 2 μM PA / OA group and sorafenib 6 μM PA / OA group. After the cells adhered to the wall, they were treated with DMSO, 2 μM sorafenib and 6 μM sorafenib for 3 h, and then treated with 0.2 / 0.4 mM PA / OA for 12 h. BSA treatment was used as a control for oil red O staining. The staining result is as figure 1 : Compared with the control group (DMSO group), the red fat droplets in the cells of the inhibitor pretreatment group were significantly reduced. The results indicated that Sorafenib significantly inhibited lipid accumulation in L02 cells, and significant changes could be seen when the pretreatment concentration of Sorafenib was 2 μmol.
Embodiment 2
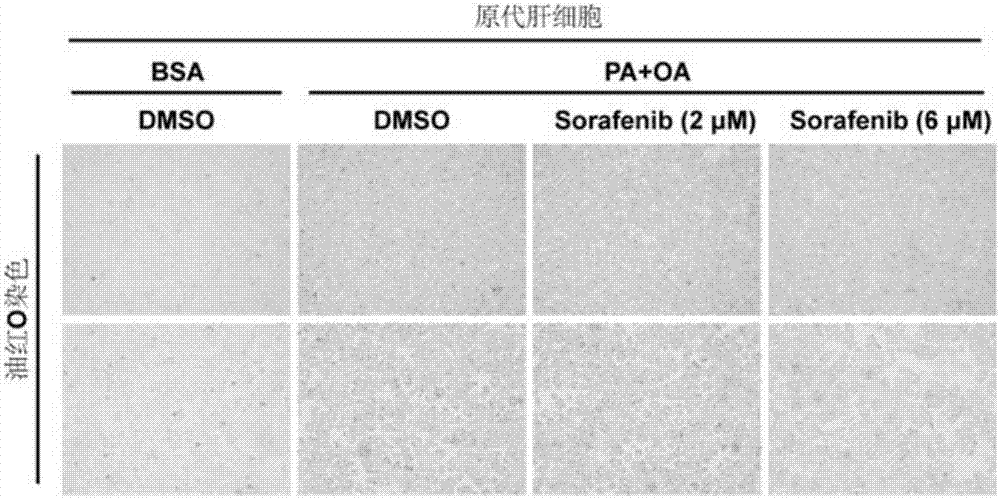
[0066] [Example 2] Effect of Sorafenib Pretreatment on Fat Accumulation in Primary Hepatocytes of Mouse
[0067] The mouse primary hepatocytes were divided into 4 groups, namely DMSO BSA group, DMSO PA / OA group, sorafenib 2 μM PA / OA group and sorafenib 6 μM PA / OA group. After the cells adhered to the wall, they were treated with DMSO, 2 μM sorafenib, and 6 μM sorafenib for 3 h, and then treated with 0.2 / 0.4 mM PA / OA for 12 h. BSA treatment was used as a control for oil red O staining. The staining result is as figure 2 : Compared with the DMSO group, the amount of red fat droplets in the inhibitor pretreatment group was significantly reduced. The results indicated that fat accumulation in L02 cells was significantly inhibited after pretreatment with sorafenib inhibitor. When the sofepramine was 6 μmol, the significant inhibitory effect of sofepramine on lipid accumulation could be detected in primary mouse hepatocytes.
Embodiment 3
[0068] [Example 3] Effect of Sorafenib on lipid accumulation in mouse liver
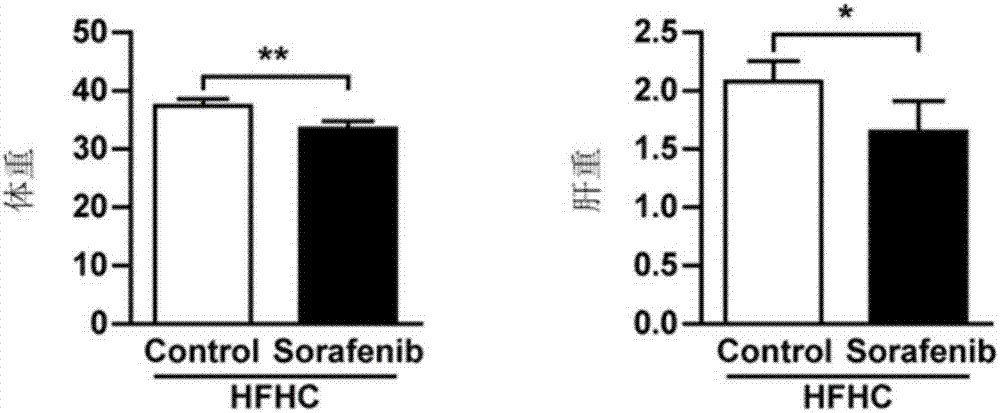
[0069] C57 male mice (20 in total) were fed with high-fat and high-cholesterol (HFHC) for 15 weeks (to establish NASH model) and were divided into two groups (10 in each group, respectively, the administration group and the control group). / kg) and vehicle (DMSO: Solutol: PEG400: water = 5:10:20:65 (v:v:v:v)) (dosing frequency: once every other day), while continuing to feed HFHC for 4 weeks, then take samples. The liver weights of the two groups of mice were measured, and oil red O staining, H&E staining and PSR staining were performed on the livers of the mice.
[0070] The results of the changes in the body weight and liver weight of the two groups of mice were as follows: image 3 As shown, the body weight and liver weight of mice administered with soferanib were significantly lower than those of mice in the control group. pass Figure 4-6 The oil red O staining, H&E staining, and PSR staining...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com