Polymer with high-efficient drug loading performance, and preparation method and application thereof
A technology for polymers and active drugs, which is applied in the field of polymers with high-efficiency drug-carrying properties and their preparation, can solve the problems of reducing rapid intermolecular aggregation of drugs, complex synthesis steps, difficult clinical applications, and the like, and achieves excellent drug-carrying properties, Simple to prepare and easy to promote
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0068] The polymer preparation method of formula (I) of the present invention follows the steps below:
[0069] In anhydrous N,N-dimethylformamide and dichloromethane mixed solvent (v:v is 1:9), using the primary amino group in polyethylene glycol with formula (II) and formula (III) Initiate the polymerization of γ-benzyl-L-aspartic acid ester-N-internal carboxylic acid anhydride. After the reaction is completed, an excessive amount of acetic anhydride can be added to end-block to obtain a polymer with a protective group, and then in N,N- Dimethylformamide neutralizes ethanolamine and reacts to obtain a copolymer with the structure of formula (IV).
[0070] In the process of preparing polymers with protective groups, the compound with the structure of formula (II) or formula (III) and the γ-benzyl-L-aspartic acid ester-N-internal carboxylic acid anhydride mono The molar ratio of the body is preferably 1:5~200, more preferably 1:10~50; the temperature of the reaction is prefer...
Embodiment 1
[0094] Add 5.00 g of polyethylene glycol monomethyl ether with a number average molecular weight of 5000 and a structure of formula (II) into the dry reaction flask, and remove water by azeotroping with 80 mL of anhydrous toluene at 130°C for 3 hours, then vacuum dry The remaining toluene; the obtained solid was dissolved in 20 mL of dry dichloromethane to obtain the first solution; 5.50 g of γ-benzyl-L-aspartate-N-endocarboxylic anhydride was dissolved in 5 mL of dry In a mixed solvent of N,N-dimethylformamide and 50 mL of dichloromethane, the second solution was obtained; in a nitrogen atmosphere, the first solution and the second solution were mixed, and stirred and reacted at room temperature under nitrogen protection conditions for 48 hours; Then 10 mL of acetic anhydride was added to continue the reaction for 24 h. After the reaction is over, remove most of the solvent under reduced pressure, then settle with ether, filter with suction, and dry to obtain a block copolyme...
Embodiment 2
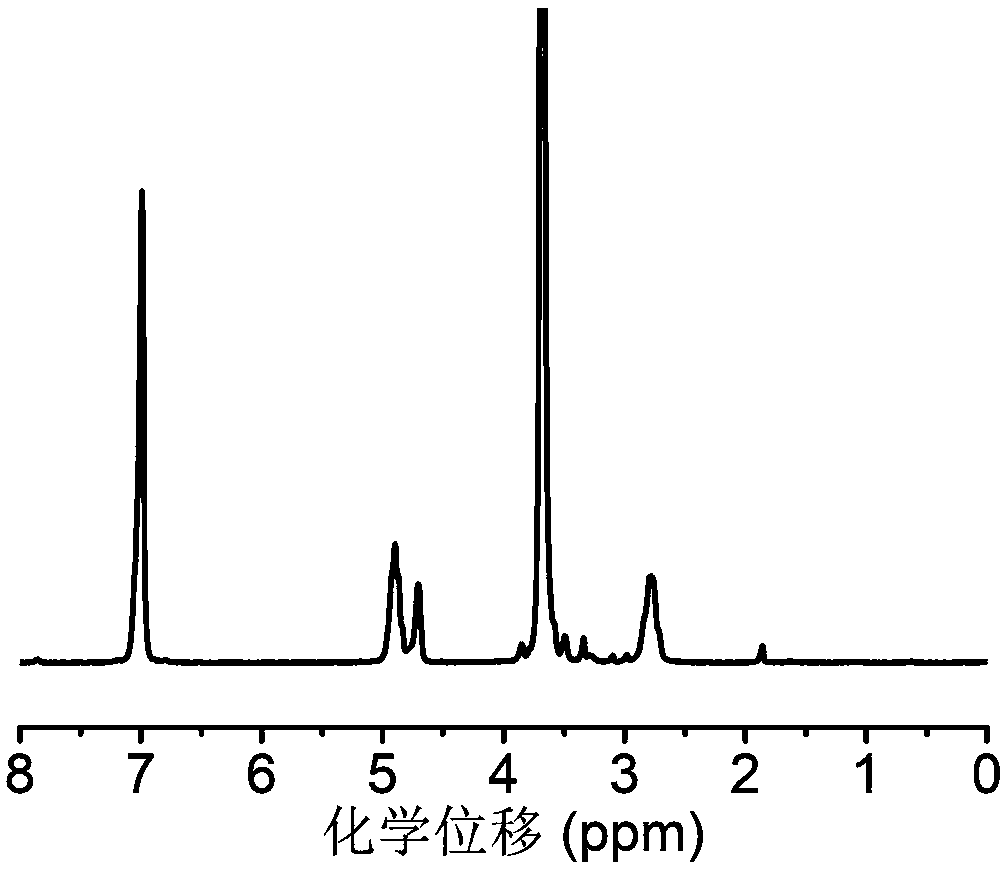
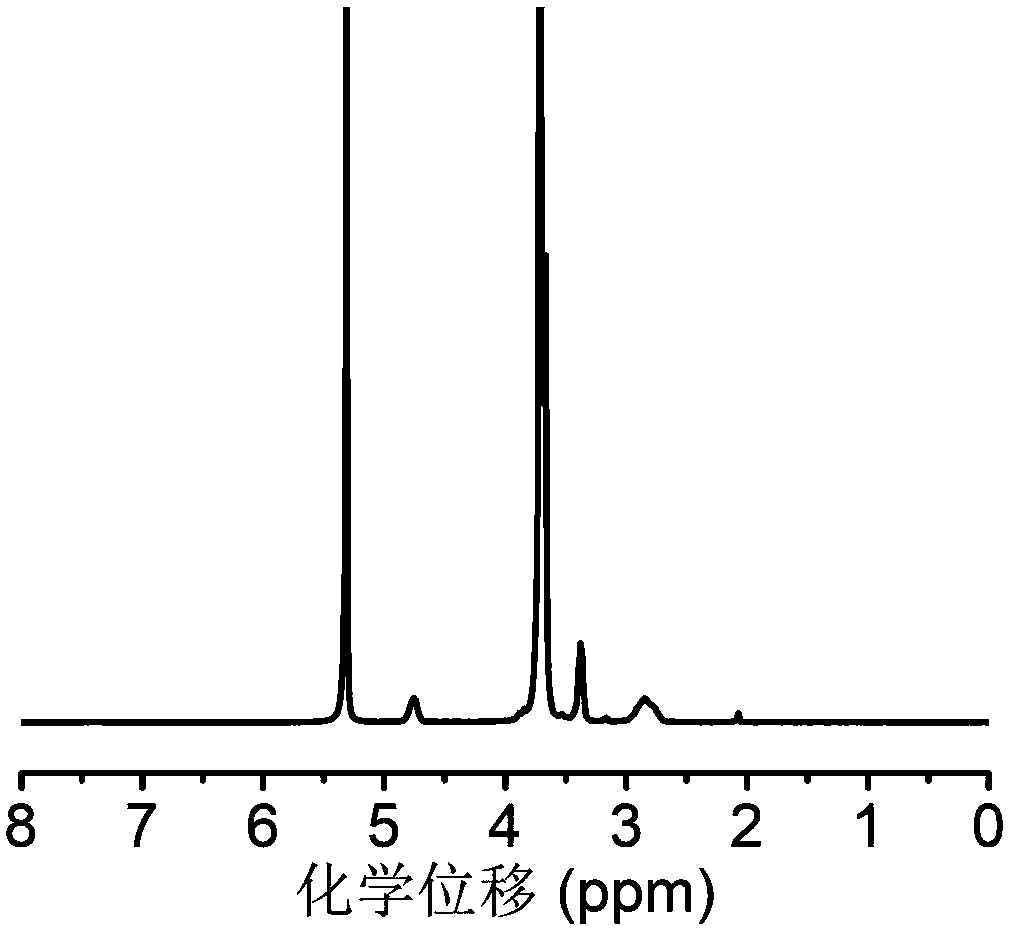
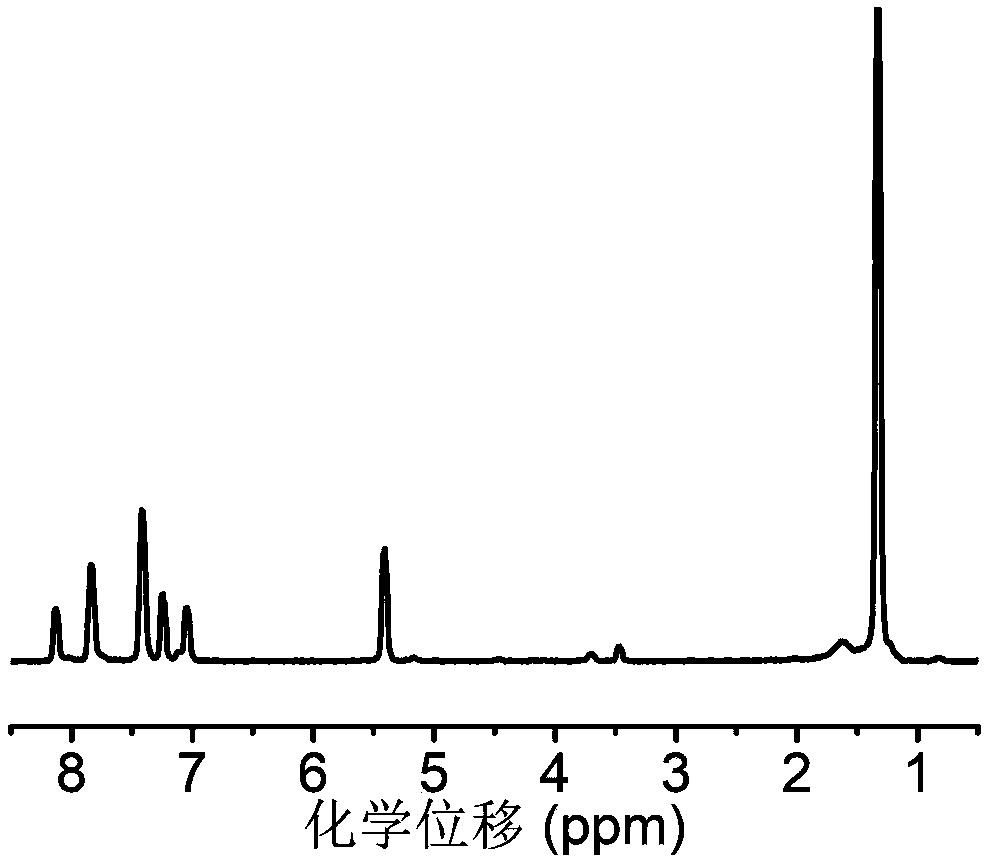
[0100] In a dry round bottom flask, add 7.2g of 4-hydroxymethylphenylboronic acid pinacol ester and 10.3g of carbonyldiimidazole, add 50mL of anhydrous dichloromethane to dissolve, and react at room temperature for 12h. After the reaction was completed, 500 mL of ethyl acetate was added to the system for dilution, and the organic layer was washed with distilled water and saturated brine successively, and then the organic layer was washed with anhydrous MgSO 4 Let dry overnight. The organic solvent was removed under reduced pressure to finally obtain 7.6 g of the small molecule of formula (V). NMR analysis was carried out to the obtained block copolymer, image 3 For the small molecule prepared in Example 2, use deuterated dimethyl sulfoxide as a solvent, and the results show that the small molecule was successfully synthesized, and its specific structure is as follows:
[0101]
[0102] Formula (V).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com