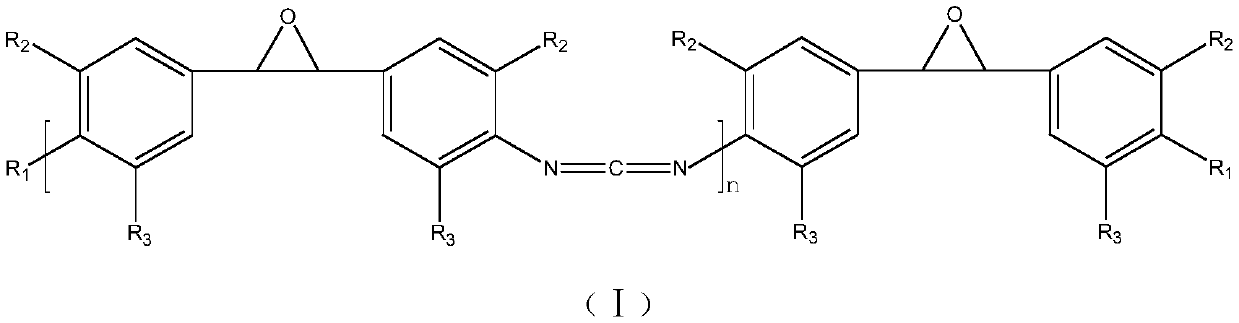
A kind of polymeric carbodiimide compound and preparation method thereof
A carbodiimide and compound technology, which is applied in the field of polymerized carbodiimide compounds and their preparation, can solve the problems of inconvenient use, difficult crystallization and purification, etc., so as to increase thermal degradation temperature, rigidity and resistance hydrolytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
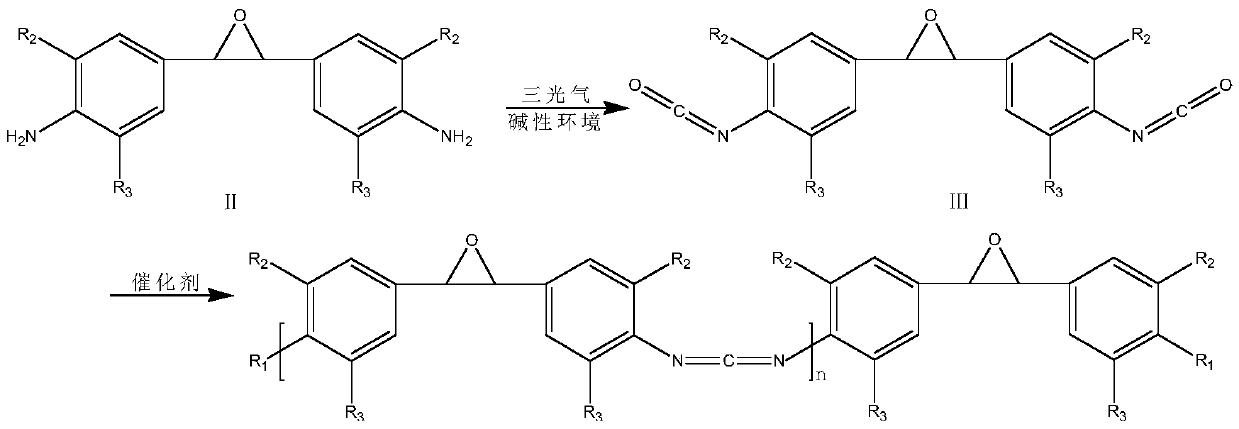
[0029] (1) Substitution reaction: add 154g triphosgene and 500ml dichloroethane in the reaction kettle, after the triphosgene is completely dissolved, add 260g anhydrous sodium bicarbonate and 500ml deionized water successively, reduce the temperature of the whole system to -2 ℃, 250g of diamine (II) was dissolved in 500ml of dichloroethane and added dropwise to the reaction kettle, reacted at low temperature for 2h, heated up to 80°C and reacted for 2h, separated into liquids, washed with water, and rotary evaporated to obtain 290g of diisocyanate (III );
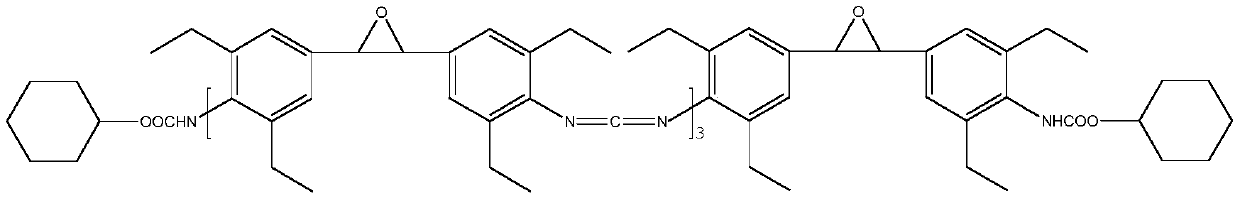
[0030] (2) Condensation reaction: At 180°C, add 0.75g of catalyst 3-methyl-1-phenyl-2-phosphorus-1-oxide to the diisocyanate (Ⅲ) obtained in the above step (1), the condensation reaction is 5 Add 65g end-blocking agent cyclohexanol after one hour, obtain 329.75g product;
[0031] (3) Product purification: at 160 DEG C, vacuum distillation under the condition of pressure 100pa, the product obtained in the above step (2) is...
Embodiment 2
[0035] (1) Substitution reaction: Add 308g of triphosgene and 1L of dichloroethane into the reaction kettle. After the triphosgene is completely dissolved, add 520g of anhydrous sodium bicarbonate and 1L of deionized water in turn, and reduce the temperature of the entire system to - At 5°C, dissolve 500g of diamine (II) in 1L of dichloroethane and add it dropwise to the reaction kettle, react at low temperature for 1h, heat up to 50°C and react for 3h, separate liquid, wash with water, and rotary evaporate to obtain 580g of diisocyanate (Ⅲ);
[0036] (2) Condensation reaction: At 120°C, add 1.5g of catalyst 1,3-dimethyl-2-phosphorus-1-oxide to the diisocyanate (Ⅲ) obtained in the above step (1), and conduct condensation reaction for 10 hours After adding 42g end-blocking agent phenyl isocyanate, obtain 547.5g product;
[0037] (3) Product purification: Distill under reduced pressure at 160°C and a pressure of 100pa, purify the product obtained in the above step (2) to obtain...
Embodiment 3
[0041](1) Substitution reaction: add 462g of triphosgene and 1.5L dichloroethane in the reaction kettle, after the complete dissolution of triphosgene, add 720g of anhydrous sodium bicarbonate and 1.5L of deionized water successively, lower the temperature of the whole system To 0°C, dissolve 750g of diamine (II) in 1.5L of dichloroethane and add it dropwise into the reaction kettle, react at low temperature for 1.5h, raise the temperature to 40°C, react for 4h, separate liquid, wash with water, and rotary evaporate to obtain 870g Diisocyanate (Ⅲ);
[0042] (2) Condensation reaction: At 200°C, add 2.25g of catalyst 1-ethyl-2-phosphorus-1-oxide to the diisocyanate (Ⅲ) obtained in the above step (1), add 102g of catalyst after 15 hours of condensation reaction End-blocking agent polyethylene glycol-methyl ether (Mw=350), to obtain 878.25g of product;
[0043] (3) Product purification: at 160 DEG C, underpressure distillation under the condition of pressure 100pa, the product ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com