Preparation method of UV (ultraviolet) moisture dual-curable modified organic silicone polymer
A dual-curing, polymer technology used in adhesives and other directions to achieve high adhesion, soft film and high reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
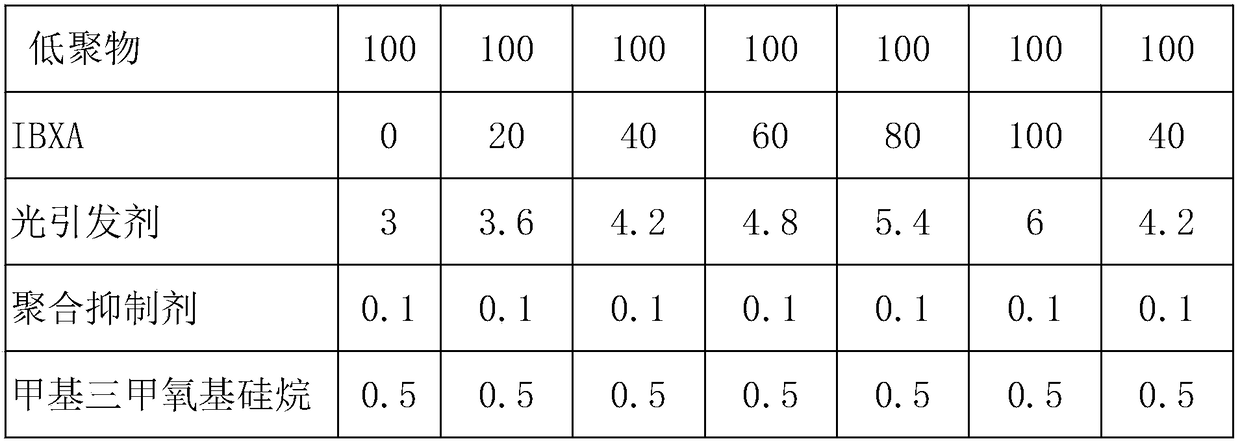
[0021] Vinyl-terminated polydimethylsiloxane polymer is prepared by the following steps: Dissolve 5.68 dihydroxymethacrylic acid with 5g of dimethylformamide, place it in a three-necked flask, and add 1 mole of silanol to seal The end polydimethylsiloxane (viscosity 1000 centipoise, molecular weight 25000) and 2.2 moles of ethyl aminopropyl trimethoxysilane and 0.05 g of bismuth octoate were condensed in a vacuum for 5 hours to remove the by-products of methanol. . Then add a certain stoichiometric amount of 2-isocyanatoethyl methacrylate, and stir under nitrogen for 30 minutes to obtain a polydimethylsiloxane oligomer with vinyl end groups. Isobornyl acrylate monomer (IBX-A) for the oligomer obtained above, photoinitiator, dibutyltin dilaurate (DBTDL) moisture curing catalyst, methyltrimethoxysilane dehydrating agent and radical polymerization inhibitor Stir in a nitrogen atmosphere to obtain the adhesive. The formula table is as follows:
[0022]
[0023]
Embodiment 2
[0025] Dissolve 2.68 dihydroxymethacrylic acid with 3g dimethylformamide, place it in a three-necked flask, add 0.5 moles of silanol-terminated polydimethylsiloxane (viscosity 2000 cps, molecular weight 26000) and 1.2 moles Butyl-substituted aminopropyltrimethoxysilane and 0.02g bismuth octoate were condensed in vacuum for 3 hours to remove the by-products of methanol. Then a certain stoichiometric amount of 2-isocyanatoethyl methacrylate was added, and Under the condition of nitrogen filling, under stirring for 60 minutes, a polydimethylsiloxane oligomer with vinyl end groups is obtained. The oligomer obtained above is used with isobornyl acrylate monomer (IBX-A), light Initiator, dibutyltin dilaurate (DBTDL) moisture curing catalyst, methyltrimethoxysilane dehydrating agent and free radical polymerization inhibitor are stirred under nitrogen atmosphere to obtain the adhesive. The formula table is as follows:
[0026] Component weight
Embodiment 3
[0028] Use 6g of dimethylformamide to dissolve 3-dihydroxymethacrylic acid, place it in a three-necked flask, add 0.5 moles of silanol-terminated polydimethylsiloxane (viscosity 1500 centipoise, molecular weight 15000) and 10.8 moles Butyl-substituted aminopropyltrimethoxysilane and 0.06g of bismuth octoate were condensed in a vacuum for 2 hours to remove the by-products of methanol. Then a certain stoichiometric amount of 2-isocyanatoethyl methacrylate was added, and Under the condition of nitrogen filling, under stirring for 60 minutes, a polydimethylsiloxane oligomer with vinyl end groups is obtained. The oligomer obtained above is used with isobornyl acrylate monomer (IBX-A), light Initiator, dibutyltin dilaurate (DBTDL) moisture curing catalyst, methyltrimethoxysilane dehydrating agent and free radical polymerization inhibitor are stirred under nitrogen atmosphere to obtain the adhesive. The formula table is as follows:
[0029] Component weight
F-1
F-2
F-3
F-4
F-5
F-6
F-7
Ol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com