Isolated antibody or antigen-binding fragment thereof and application of isolated antibody or antigen-binding fragment thereof in tumor therapy
A technology that combines fragments and antigens, applied in anti-tumor drugs, anti-receptors/cell surface antigens/cell surface determinant immunoglobulins, antibodies, etc., can solve problems such as lack of immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0203] Example 1: Obtaining of mouse anti-human OX40 activating monoclonal antibody
[0204] The inventors of the present application constructed a CHO cell line overexpressing OX40, used it to immunize mice, and performed hybridoma cell fusion. Through antigen-antibody binding experiments and cell function screening, a series of murine antibodies with strong ELISA binding activity and OX40 activation function were obtained. The sequences of the variable regions VH and VL of each antibody were obtained by sequencing, and human-mouse chimeric antibodies were designed and expressed accordingly. The heavy chain variable region was selected from the following sequences: SEQ ID NO: 1, SEQ ID One or more of NO:2, SEQ ID NO:3, SEQ ID NO:7, SEQ ID NO:9, SEQ ID NO:10 and SEQ ID NO:11. The light chain variable region is selected from the following sequences: SEQ ID NO: 4, SEQ ID NO: 5, SEQ ID NO: 6, SEQ ID NO: 8, SEQ ID NO: 12, SEQ ID NO: 13 and SEQ ID NO : One or more of 14.
[0205...
Embodiment 2
[0215] Embodiment 2: ELISA binding experiment
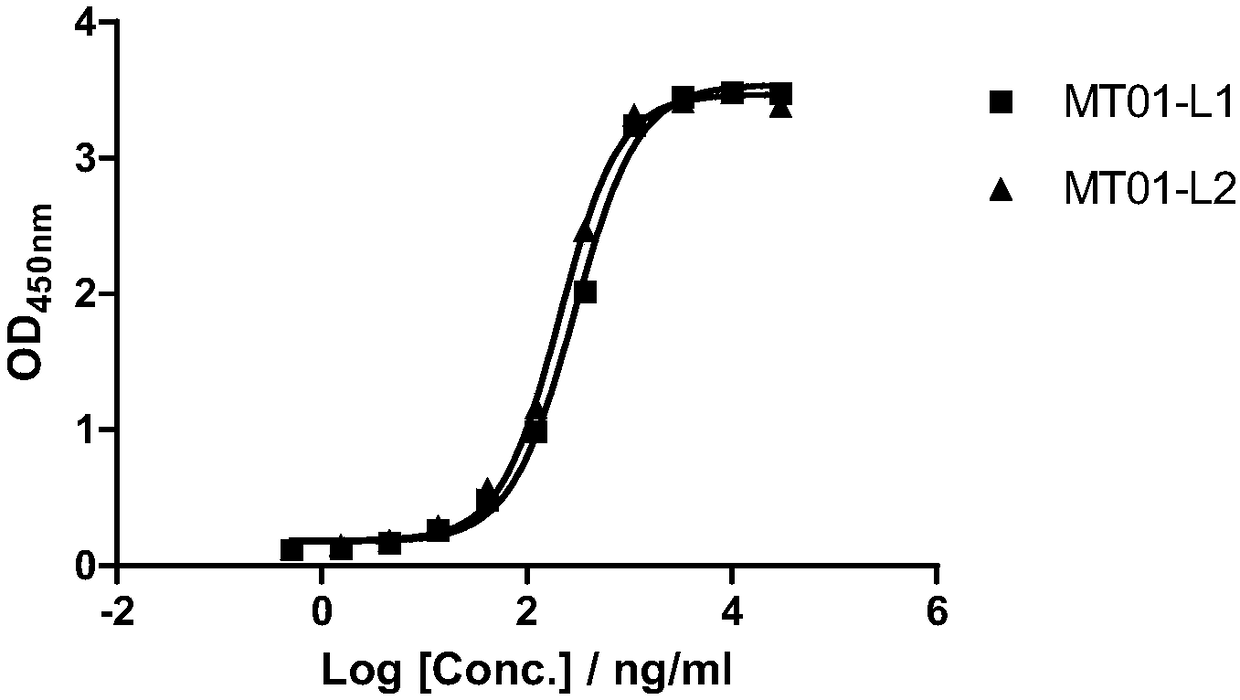
[0216] The protein solution with a concentration of 1 μg / mL was coated on a 96-well high-affinity plate at 100 μL / well, and shaken overnight at 4 °C. The next day, wash 3 times with 300 μL PBST (Tween20: 0.5‰), then block with 100 μL / well of 5% BSA / PBS for 2 hours, and shake at room temperature. Wash 3 times with 300 μL PBST. Prepare serial dilutions of samples in PBS. Add 100 μL / well to a 96-well plate and shake at room temperature for 1 hour. Wash 3 times with 300 μL PBST. Prepare the secondary antibody goat anti-human IgG HRP solution, add 100 μL / well to the 96-well plate, and shake at room temperature for 1 hour. Wash 4 times with 300 μL BST. Add 100μL / well TMB, develop color for 20min. Add 100 μL / well 0.6N H2SO4 to stop color development and detect OD450nm.
[0217] After testing, the results of figure 1 As shown, the ELISA binding EC50 of human-mouse chimeric antibodies MT01-L1 and MT01-L2 are 290.7ng / mL and 208.5ng...
Embodiment 3
[0218] Example 3: Binding to CHO-hOX40
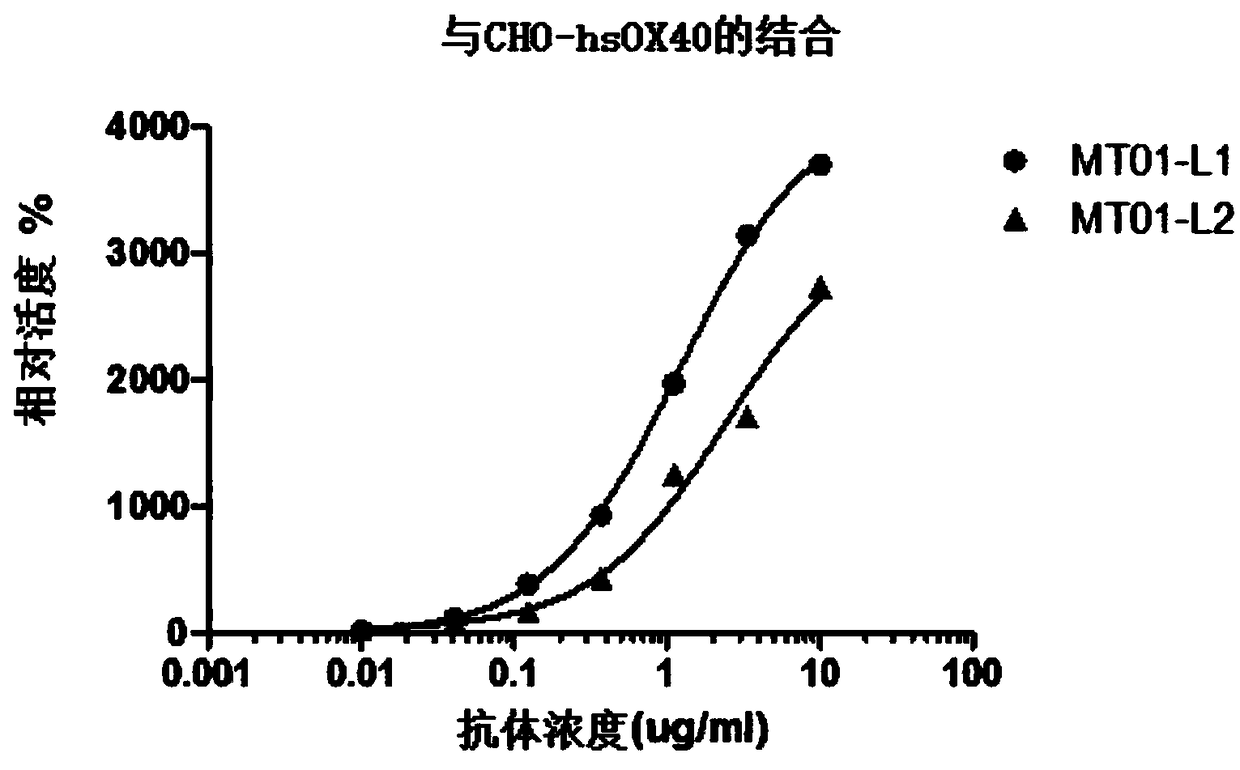
[0219] Use PBS to prepare the OX40 antibody gradient, and prepare a final concentration of 10× working solution. Collect CHO-hOX40 cells, wash once with PBS, count, and dilute to 2*10^6 / ml cell suspension; add 10 μl OX40 antibody working solution to 100 μl cell suspension, and incubate at 4°C in the dark for 30 minutes; wash with PBS twice , add secondary antibody, incubate at 4°C in the dark for 30 min, wash once with PBS, suspend with 400 μl FACS buffer, and detect on the machine. Such as figure 2 As shown, the results show that MT01-L1 and MT01-L2 bind to human OX40, and their EC50 are 1.22 μg / mL and 2.42 μg / mL, respectively.
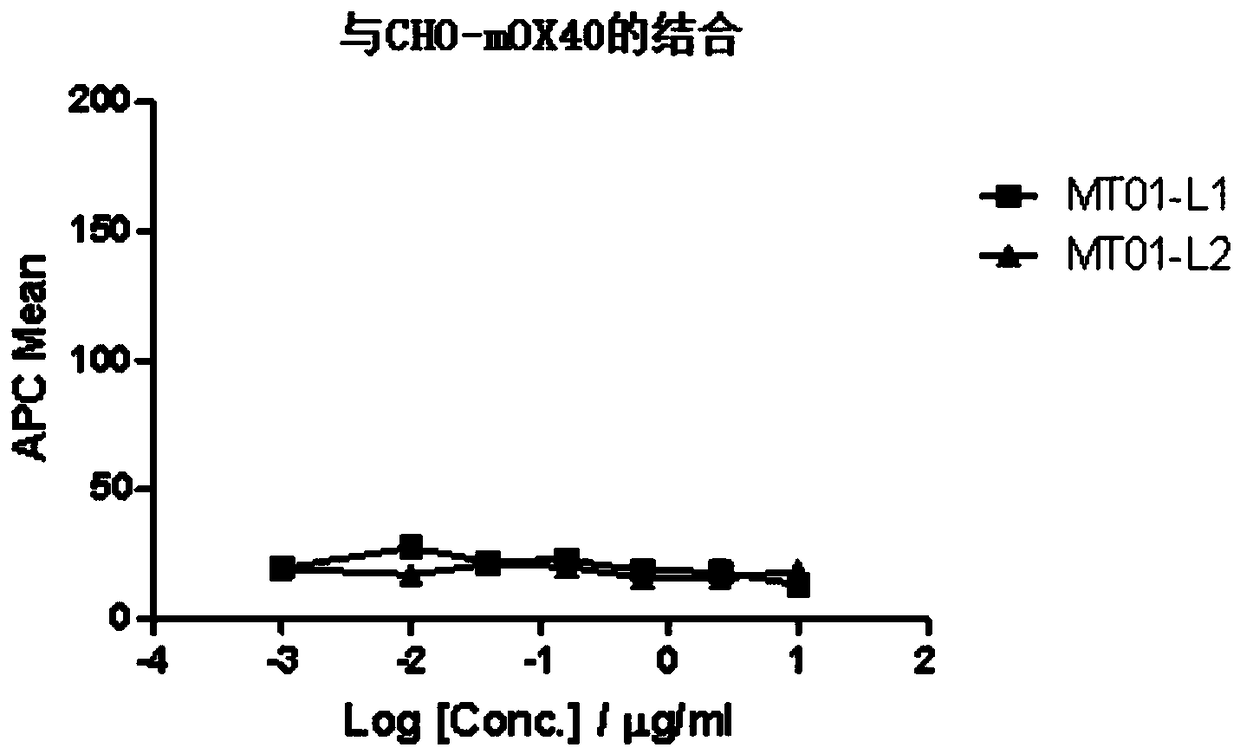
[0220] Similarly, the inventors of the present application tested the binding of antibodies to CHO cells expressing mouse OX40, see image 3 As a result of the binding experiment, it was found that neither MT01-L1 nor MT01-L2 combined with mouse OX40.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com