Noninvasive method and kit for capturing and separating fetus cells from parent
A technology for fetal and nuclear red blood cells, applied in the direction of embryonic cells, cell culture active agents, chemical instruments and methods, etc., can solve the problems of low detection rate, poor detection sensitivity, late detection window period, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
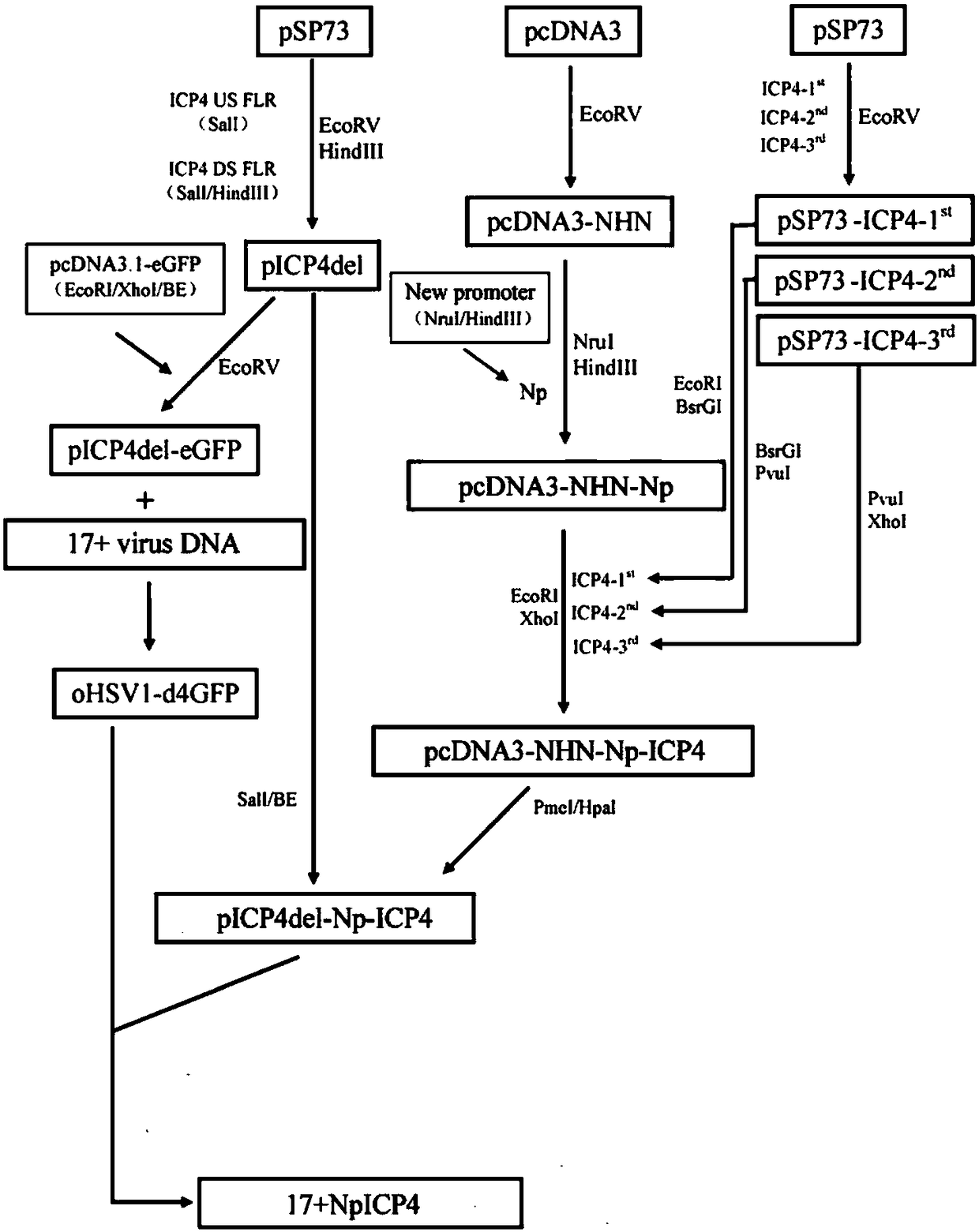
[0106] This example is used to prepare the recombinant type I herpes simplex virus of the present invention.
[0107] Purification of wild-type herpes simplex virus type I 17+ viral DNA
[0108] BHK cells were used to grow wild-type 17+ virus with DNAzol TM Genomic DNA Isolation Kit (Helena Biosciences Cat. No. DN127200) was used to purify wild-type herpes simplex virus type I 17+ virus DNA.
[0109] BHK cells were grown in a 175 cm square culture flask, and the culture medium was DMEM containing 10% fetal bovine serum and 1% penicillin and streptomycin. The culture conditions were 37°C, 5% carbon dioxide. When the cells grew to 90% confluence, wild-type HSV type I 17+ virus was inoculated. Continue to incubate for 24-48 hours. When more than 90% of the cells have cytopathic changes, remove the culture medium and add 10ml of DNAzol. Use a 10ml pipette to suck and blow 5 times, and transfer the cell lysate to a 50ml Falcon test tube, add 5ml of 100% ethanol, and shake the...
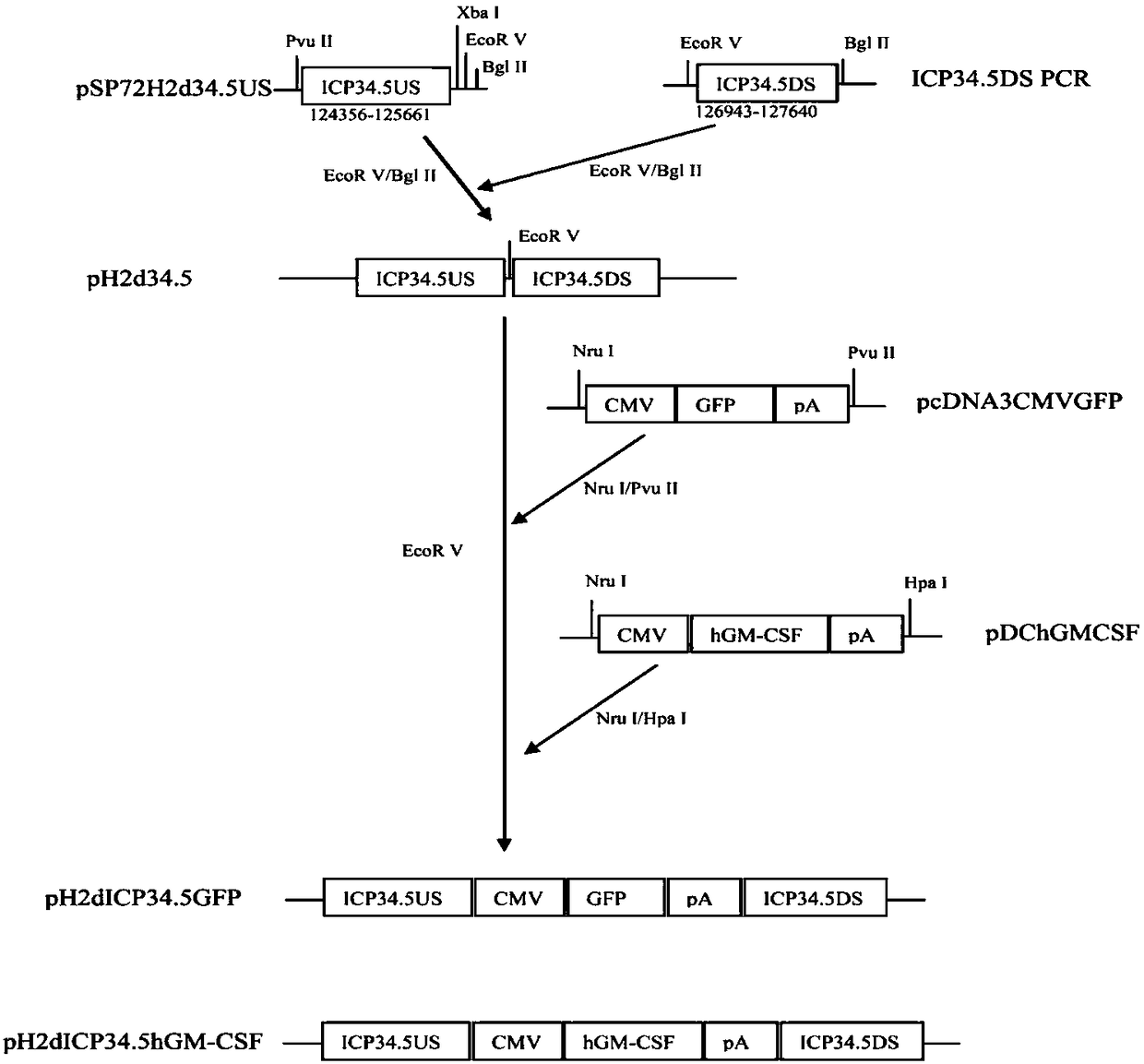
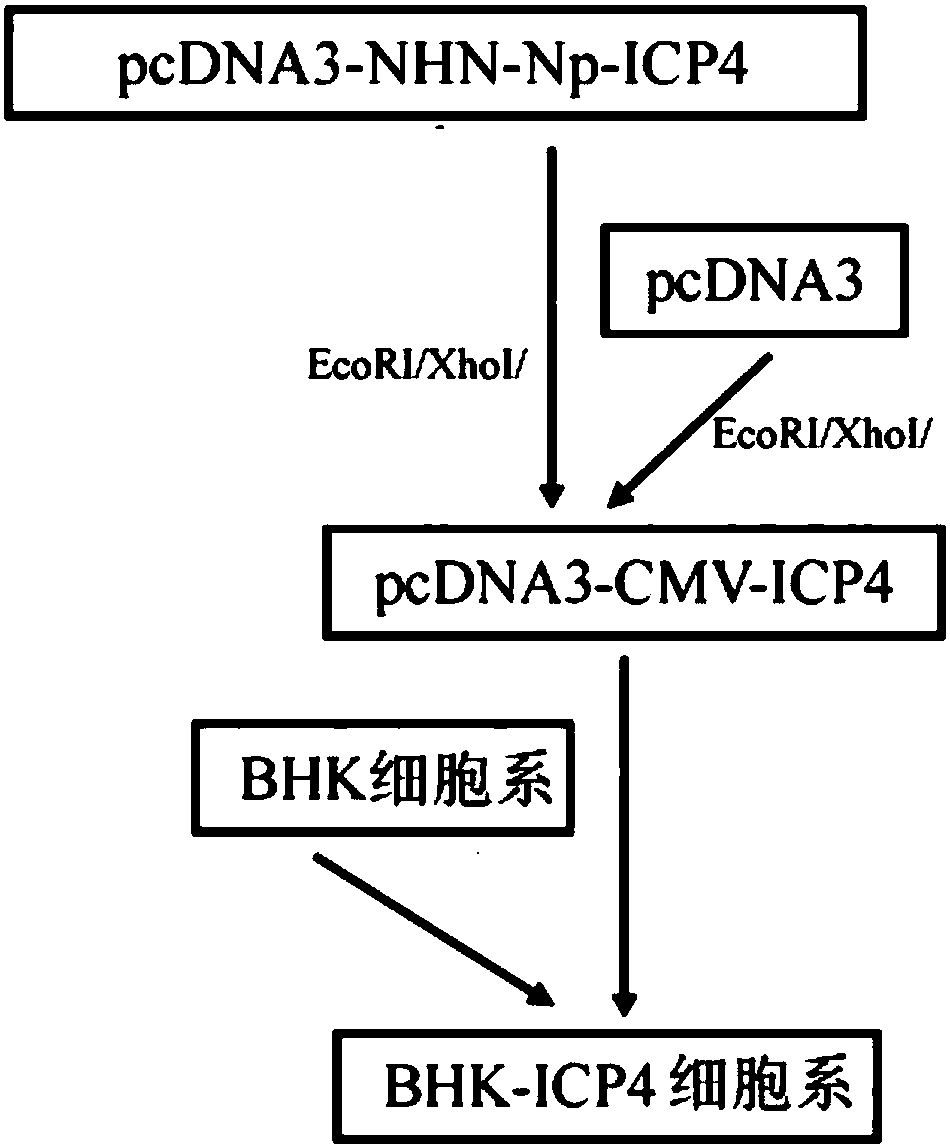
Embodiment 2
[0189] This example describes a method to specifically capture and isolate rare fetal cells.
[0190] 10 prepared by embodiment 1 10 The pfu recombinant virus solution was centrifuged at 2000rpm for 10 minutes, the supernatant DMEM medium was discarded, and the virus was suspended in RPMI-1640 medium, and the virus titer was 1×10 7 cfu of virus suspension.
[0191] The titer of the virus prepared above was 1×10 7 The virus suspension of cfu and the erythrocyte lysate that pH is 7 and the phosphate buffer that pH is 7.3 form the fetal cell capture and separation diagnostic kit that is used in following embodiment 3 and embodiment 6, wherein, described erythrocyte lysate consists of 0.15M Ammonium Chloride, 10nM Potassium Bicarbonate and 1nM EDTA.
[0192] The titer of the virus prepared above was 1×10 7 The cfu virus suspension, Ficoll-Urografin with a specific gravity of 1.077±0.001 kg / m3 and a phosphate buffer with a pH of 7.3 constitute the fetal cell capture and isolati...
Embodiment 3
[0195] This example is used to describe the effectiveness and sensitivity of the fetal cell capture and isolation diagnostic kit of the present invention.
[0196] Materials and methods:
[0197] 1) Take 5ml of peripheral blood from pregnant women at 8 weeks of pregnancy in EDTA anticoagulant tube, add 45ml of red blood cell lysate, and incubate at room temperature for 10 minutes;
[0198] 2) After the erythrocytes are lysed, centrifuge (800g, 10 minutes);
[0199] 3) Remove the supernatant, resuspend the cell pellet in 10ml of phosphate buffered solution (PBS) with a pH value of 7.3, and centrifuge (800g, 10 minutes);
[0200] 4) remove the supernatant, and resuspend the cell pellet in 2ml RPMI-1640;
[0201] 5) 2ml of cells obtained by step 4) and 0.1ml of recombinant herpes virus I (PSG3 type) suspension of the present invention (10 6 Pfu / ml) were mixed and added to the wells of the 6-well culture plate;
[0202] 6) Incubate the culture plate in 5% CO 2 in a 37°C incub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com