Xanthine compounds and their pharmaceutical compositions and applications
A compound, purine technology, applied in the field of medicine, can solve problems such as affecting drug efficacy and drug loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
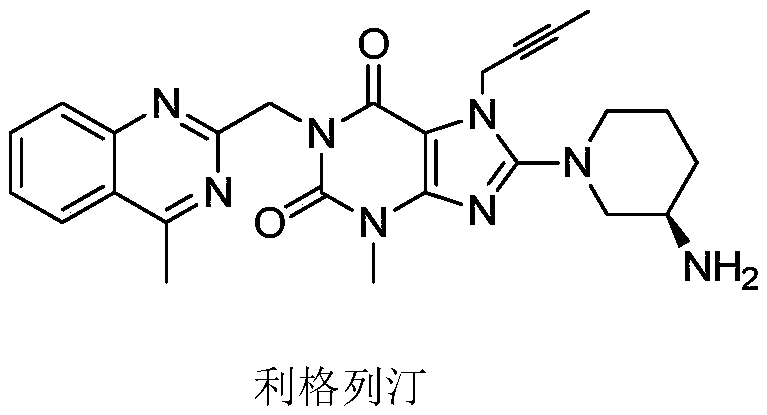
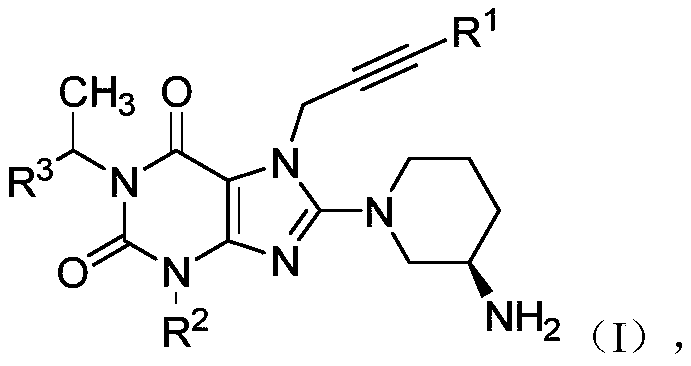
[0142] Example 1: 8-[(3R)-3-amino-1-piperidinyl]-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-[ 1-(4-Methylquinazolin-2-yl)ethyl]-1H-purin-2,6-one
[0143] Step 1) Synthesis of Intermediate II
[0144] Add sodium (11.5g, 0.5mol) and absolute ethanol (250ml) to a 1L reaction flask, stir at room temperature for 1h, add methylurea (18.6g, 0.25mol) and ethyl cyanoacetate (26.5ml, 0.25mol) , heated to reflux for 6h. The reaction solution was cooled to room temperature, and ethanol was recovered under reduced pressure. Add distilled water (50ml) to dissolve, add dropwise 4mol / L hydrochloric acid to adjust to pH7, stir in an ice bath for 1h, filter to obtain a crude product, and recrystallize with water to obtain white crystals (31.7g, 90%), ESI-MS (m / z ):164.1[M+Na] + ,305.1[2M+Na] + ; 1 HNMR (400MHz, DMSO-d 6 ): δ (ppm) 10.31 (s, 1H), 6.76 (s, 2H), 4.55 (s, 1H), 3.17 (s, 3H).
[0145] Step 2) Synthesis of Intermediate III
[0146] Intermediate II (5g, 35.45mmol), distilled wate...
Embodiment 2
[0160] Example 2: 8-[(3R)-3-amino-1-piperidinyl]-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-[ 1-(4-Nitrophenyl)ethyl]-1H-purin-2,6-one
[0161] ESI-MS(m / z):466.1[M+H] + ,488.3[M+Na] + ,953.3[2M+Na] + ; 1 H-NMR (400MHz, CDCl 3 ): δ (ppm) 8.15 (d, J = 8.8Hz, 2H), 7.55 (d, J = 8.4Hz, 2H), 6.42 (q, J = 6.8Hz, 1H), 4.84 (s, 2H), 3.71 –3.63(m,1H),3.60–3.53(m,1H),3.43(s,3H),3.12–3.02(m,2H),2.93–2.83(m,1H),2.04–1.95(m,1H) ,1.90(d,J=6.8Hz,3H),1.82(s,3H),1.42(m,1H),1.40–1.22(m,1H),0.92–0.80(m,1H); 13 C-NMR (100MHz, CDCl 3 ): δ (ppm) 156.5, 154.1, 150.9, 149.0, 148.2, 146.6, 127.7, 123.3, 104.2, 81.5, 73.0, 58.1, 50.3, 47.3, 35.8, 33.4, 29.5, 26.9, 23.3, 22.6, 15.8, 14.1, 3.7.
Embodiment 3
[0162] Example 3: 8-[(3R)-3-amino-1-piperidinyl]-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-[ 1-(Phenylethyl)]-1H-purin-2,6-one ESI-MS(m / z):421.2[M+H] + ; 1 H-NMR (400MHz, CDCl 3 ):δ(ppm)7.51–7.10(m,5H),5.26(q,J=5.8Hz,1H),4.79(s,2H),4.34–4.25(m,1H),4.22–4.16(m,1H ),4.09(s,3H),3.95–3.83(m,2H),3.78–3.70(m,1H),3.09–3.00(m,1H),2.94(d,J=5.6Hz,3H),2.90( s,3H),2.86–2.79(m,1H),2.57–2.49(m,1H),2.18–2.09(m,1H); 13 CNMR (100MHz, CDCl 3 ): δ (ppm) 156.1, 154.3, 151.5, 147.6, 144.2, 128.4, 127.3, 126.1, 104.5, 81.3, 73.2, 68.4, 58.2, 50.5, 47.3, 41.1, 33.4, 29.6, 27.4, 24.9, 24.3, 23.3, 3.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com