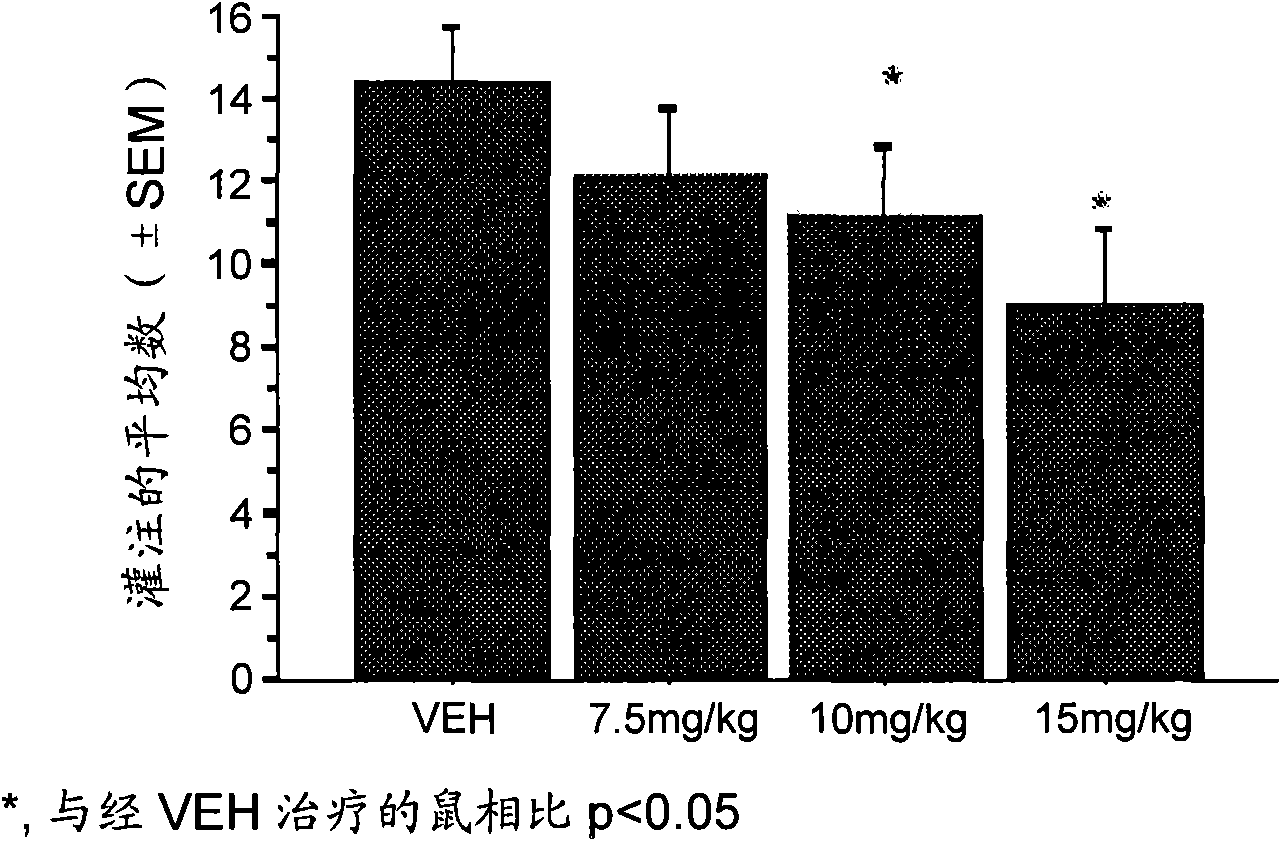
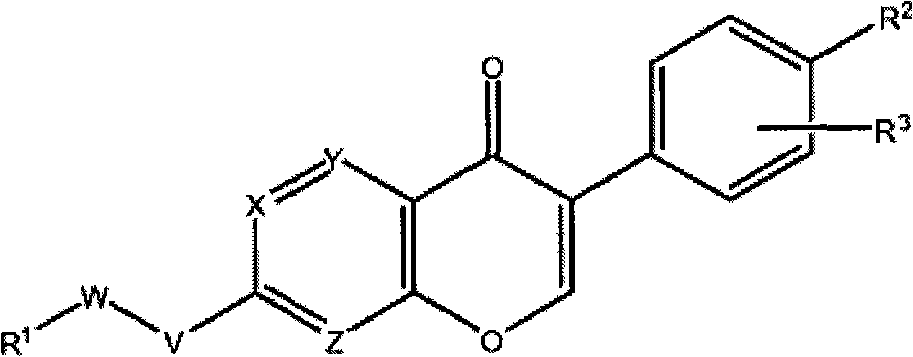
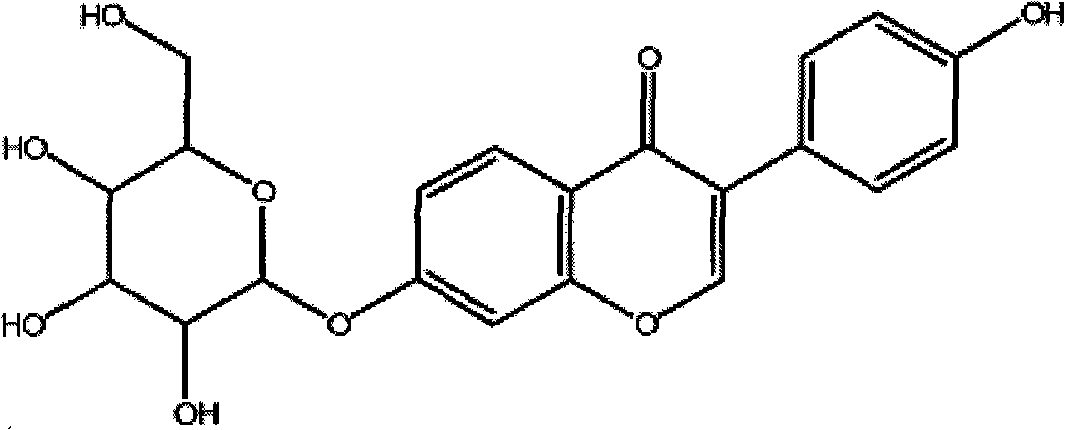
Aldh-2 inhibitors in the treatment of addiction
A technology of R20 and compounds, applied in the field of novel ALDH-2 inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0341] Step 1-Preparation of the compound of formula (2)
[0342] Usually, where R 1 The mixture of the compound of formula I which is benzonitrile (or benzonitrile), dibutyltin oxide, and trimethyl azide is subjected to microwave treatment. The reaction is carried out at a temperature of about 150°C for about 10-30 minutes. When the reaction is substantially complete, the product of formula (2) is separated by conventional means, such as by chromatography (or chromatography) on silica gel.
[0343] Step 2-Preparation of Compound of Formula I
[0344] The purified product of formula (2) is suspended in an aqueous solvent, such as acetonitrile / water, and a catalytic amount of a strong acid, such as trifluoroacetic acid, is added. Removal of solvent provides where R 1 The compound of formula I which is a phenyl substituted by tetrazol-5-yl.
[0345] Similarly, where R 1 The compound of formula I which is [1,2,4]-oxadiazol-3-yl substituted by benzonitrile at position 5 is converted t...
Embodiment 1
[0454] Preparation of the compound of formula (4)
[0455] A. Preparation of compounds of formula (4) where R is phenyl
[0456]
[0457] A 50 mL round bottom flask equipped with a condenser was filled with benzamide (compound of formula (b), 363.4 mg, 3.0 mmol) and 1,3-dichloroacetone (457.1 mg, 3.6 mmol, 1.2 equivalents). The mixture was heated at 130°C for 1 hour under nitrogen. After cooling to room temperature, the resulting mixture was purified from acetonitrile (6 mL) by recrystallization. The suspension was heated for 5 minutes under reflux reaction conditions and cooled to ambient temperature. The obtained solid was filtered through a glass filter, and the crystals on the filter were rinsed with acetonitrile (2 mL). The desired product, 4-(chloromethyl)-2-phenyl-1,3-oxazole (285.8 mg, 1.48 mmol, 49%) was obtained as a colorless powder.
[0458] B. Preparation of other compounds of formula (4a) where R is phenyl
[0459] Similarly, following the procedure of Example IA,...
Embodiment 2
[0489] Preparation of the compound of formula (5)
[0490]
[0491] step 1- Preparation of the compound of formula (9)
[0492] A mixture of 1-(2-hydroxy-4-methoxyphenyl)ethan-1-ol (20g, 120mmol) and N,N-dimethylformamide dimethyl acetal (23g, 181mmol) Stir at 90°C for 2 hours. After cooling to room temperature, the reaction mixture was provided with a yellow precipitate, which was washed with ethyl acetate (3x30ml), water (2x50ml), and dried under reduced pressure to produce 3-(dimethylamino) as the trans isomer -1-(2-hydroxy-4-methoxyphenyl)prop-2-en-1-one (9); MS 222.1(M+H)
[0493] Step 2- Preparation of the compound of formula (5)
[0494] To 3-(dimethylamino)-1-(2-hydroxy-4-methoxyphenyl)prop-2-en-1-one (20.0g, 100ml) in anhydrous chloroform (100ml) at 0°C 90.37mmol) was added N-iodosuccinimide (23.5g, 99.22mmol) and silica gel (40g). The reaction mixture was stirred at 0°C for 60 minutes, and then insoluble materials were filtered off. The filtrate was rinsed with aqueo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com