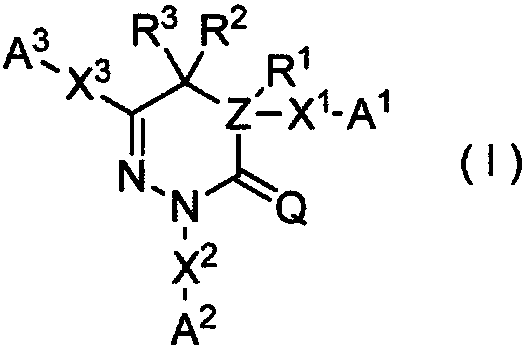
Pyranodipyridine compound
A compound and bipyridine technology, applied in the field of pyranodipyridine compounds, can solve the problems of reducing the quality of life of epilepsy patients, limiting the dosage, reducing the inhibitory effect of the central nervous system, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0245] Compounds (I) to (XXII) of the present invention can be produced, for example, by the methods described in the following examples, and the effects of the compounds can be confirmed by the methods described in the following test examples. It should be noted, however, that these examples are illustrative, and the present invention is in no way limited to the following specific examples, and furthermore, these examples can be modified without departing from the scope of the present invention.
[0246] The compound described with the name of a document etc. shows that it was manufactured based on the said document etc.
[0247] The abbreviations used in this specification are commonly used abbreviations known to those skilled in the art. In this specification, the following abbreviations are used.
[0248] AIBN: 2,2'-Azobis(isobutyronitrile)
[0249] (Ataphos) 2 PdCl 2 : Bis(di-tert-butyl(4-dimethylaminophenyl)phosphine)palladium(II) dichloride
[0250]DCM: dichloromet...
example 1
[0280] Synthesis of 6H-pyrano[3,2-b:5,4-b']dipyridin-8(7H)-one
[0281] [chemical formula 56]
[0282]
[0283] (1) Synthesis of (3-bromo-6-methoxypyridin-2-yl)methyl acetate
[0284] 3-Bromo-6-methoxy-2-methylpyridine (CAS No. 126717-59-7) (4.87kg, 24.1mol, 1 equivalent) was dissolved in chloroform (25L) and cooled to 0-10 ℃. To this solution was added 65% mCPBA (8.32 kg, 31.3 mol, 1.3 eq) and the resulting suspension was heated at 40°C to 50°C for 10 hours. The reaction solution was cooled to 10°C and stirred for 15 minutes. This suspension was filtered, and the residue was washed with chloroform (20 L). The combined filtrates were dried over anhydrous sodium sulfate and filtered. To the obtained filtrate was added acetic anhydride (12.2 L, 129 mol, 5.4 equivalents) at room temperature, and heated and stirred at 65°C to 70°C for 12 hours. After the reaction, the reaction solution was cooled to room temperature. Methanol (35 L) was added to the reaction liquid, ...
example 2
[0295] Synthesis of 9-bromo-7-(pyridin-3-yl)-6H-pyrano[3,2-b:5,4-b']dipyridin-8(7H)-one
[0296] [chemical formula 57]
[0297]
[0298] (1) Synthesis of 7-(pyridin-3-yl)-6H-pyrano[3,2-b:5,4-b']dipyridin-8(7H)-one
[0299] To 6H-pyrano[3,2-b:5,4-b']dipyridin-8(7H)-one (4g, 20.0mmol, 1 equivalent) obtained in Production Example 1, silver carbonate (6.61 g, 24.0mmol, 1.2 equivalents), copper iodide (I) (2.28g, 12.0mmol, 0.6 equivalents), pyridine (9.7mL, 120mmol, 6 equivalents) and DMF (100mL) in a mixture under an oxygen environment at 65 A suspension of 1,3-propanediol pyridine-3-boronate (CAS No. 131534-65-1 ) (9.77 g, 59.9 mmol, 3 equiv) in DMF (100 mL) was added slowly at °C. The reaction mixture was stirred overnight at 65 °C. The reaction solution was cooled to room temperature, and NH silica gel was added. diatomaceous earth TM Filter and wash the residue with chloroform. The obtained filtrate was concentrated under reduced pressure. The obtained residue wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com