A kind of preparation method of 2,2'-dihydroxybenzophenone compounds
A technology for dihydroxybenzophenone and compounds, which is applied in the field of synthesis of ketone acyclic compounds, can solve the problems of high raw material requirements and expensive Grignard reagents, and achieve the effects of high purity, easy availability of raw materials, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Preparation of 5-methyl-3-[5-methyl-2-hydroxyphenyl]benzofuran-2-(3hydro)-one (p-methyllactone)
[0056] 21.6g of p-cresol (0.2 mole), 9.2g of glyoxylic acid monohydrate (0.1 mole) and 0.3 g of p-toluenesulfonic acid were placed in a 500ml there-necked flask, 200ml of toluene was added, and a magnetic stirring belt was added after the heating was fully dissolved. The water was refluxed for 6 hours, and the liquid phase followed the reaction. After the reaction was completed, the solvent was evaporated to dryness to obtain a yellow-brown solid, which was then recrystallized with methanol to obtain 22.5 g of a light-yellow solid, that is, 5-methyl-3-[5-methyl- 2-Hydroxyphenyl]benzofuran-2-(3hydro)-one, referred to as methyl lactone, has a liquid phase purity of 98.3% and a yield of 90.1%.
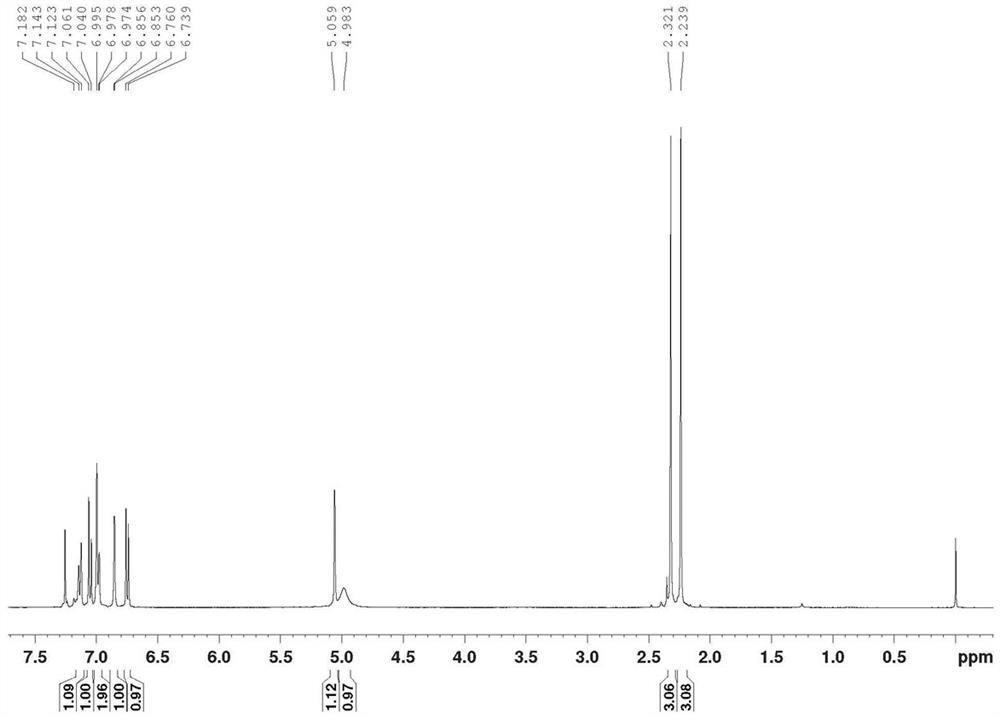
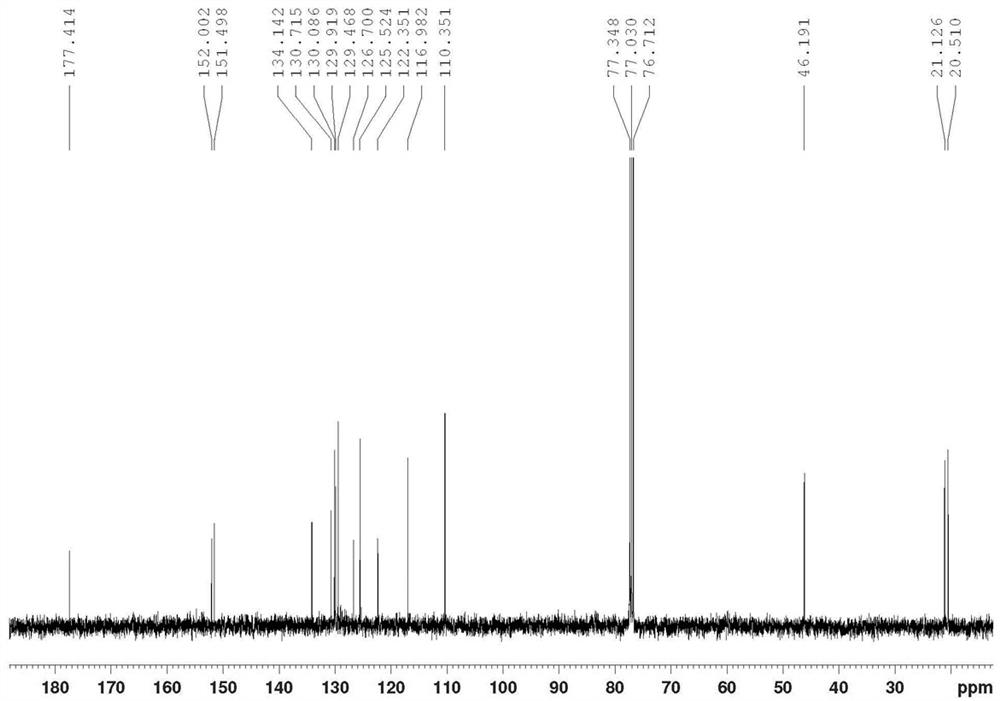
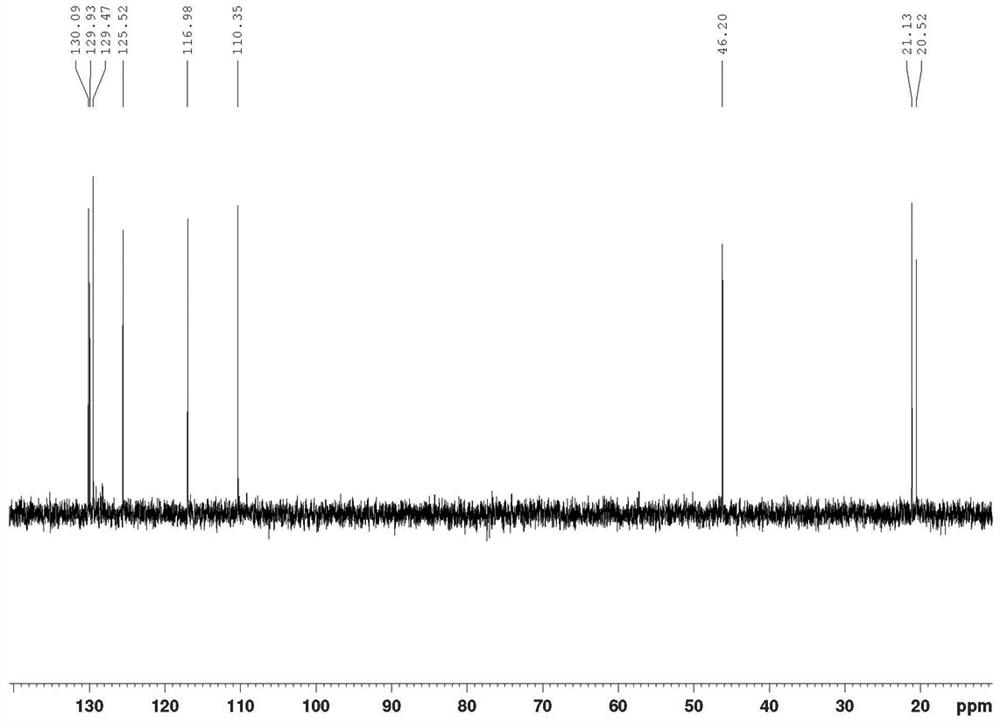
[0057] Product structure verification (see Figures 1A-1E ):
[0058] 1 HNMR(δ,ppm,400MHz,CDCl 3 ): 2.239(s,3H,CH 3 ); 2.321(s,3H,CH 3 ); 4.983(s, 1H); 5.059(s, 1H); 6...
Embodiment 2
[0063] Example 2: Preparation of 5-ethyl-3-[5-ethyl-2-hydroxyphenyl]benzofuran-2-(3hydro)-one (p-ethyllactone)
[0064] Place 24.4g p-ethylphenol (0.2 mol), 9.2g glyoxylic acid monohydrate (0.1 mol) and 0.3 gram p-toluenesulfonic acid in a 500ml there-necked flask, add 200ml toluene, and heat it with a magnetic stirring belt after it is completely dissolved. The water was refluxed for 6 hours, and the liquid phase followed the reaction. After the reaction was complete, the solvent was evaporated to dryness to obtain a yellow-brown solid, which was then recrystallized with ethanol to obtain 25.6 g of a light-yellow solid, namely 5-ethyl-3-[5-ethyl-2 -Hydroxyphenyl]benzofuran-2-(3hydro)-one, referred to as ethyl lactone, the liquid phase purity of the ethyl lactone is 98.5%, and the yield is 92.0%.
[0065] Product structure verification (see Figures 2A-2E ):
[0066] 1 HNMR(δ,ppm,400MHz,CDCl 3 ): 1.182(m, 6H, CH 3 ); 2.536(q, 2H); 2.613(q, 2H); 4.679(s, 1H); 5.081(s, 1H)....
Embodiment 3
[0071] Example 3: Preparation of 5-isopropyl-3-[5-isopropyl-2-hydroxyphenyl]benzofuran-2-(3hydro)-one (p-isopropyllactone)
[0072] Place 27.2g of p-isopropylphenol (0.2 mole), 9.2g of glyoxylic acid monohydrate (0.1 mole) and 0.3 g of p-toluenesulfonic acid in a 500ml three-necked flask, add 200ml of toluene, and stir magnetically after heating and dissolving. The reaction was carried out under reflux with water for 6 hours, and the liquid phase followed the reaction. After the reaction was completed, the solvent was evaporated to dryness to obtain a yellow-brown solid, which was then recrystallized with ethanol to obtain 27.3 g of a light-yellow solid, namely 5-isopropyl-3-[5-isopropyl] Base-2-hydroxyphenyl]benzofuran-2-(3-hydro)-one, referred to as propyl lactone, the liquid phase purity of the propyl lactone is 98.7%, and the yield is 89.2%.
[0073] Product structure verification (see Figures 3A-3E ):
[0074] 1 HNMR(δ,ppm,400MHz,CDCl 3 ): 1.170 (m, 6H); 1.227 (d, 6H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com