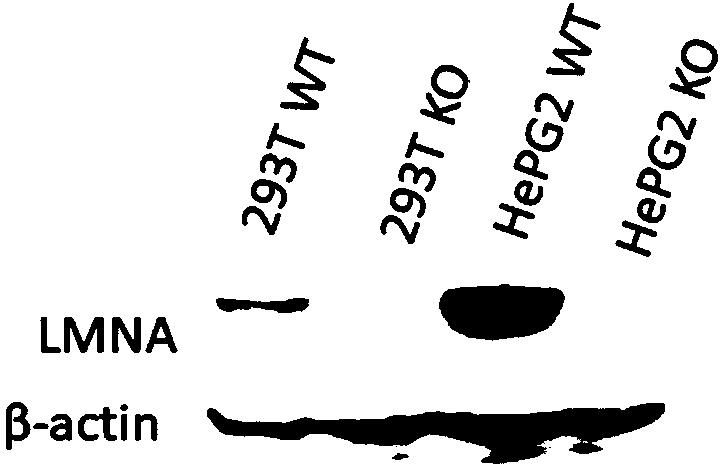
LMNA gene knocked-out cell line constructed on basis of CRISPR/Cas9 technology
A gene knockout and cell line technology, applied in the biological field, can solve the problems of lack of gene knockout cell lines and insufficient research.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
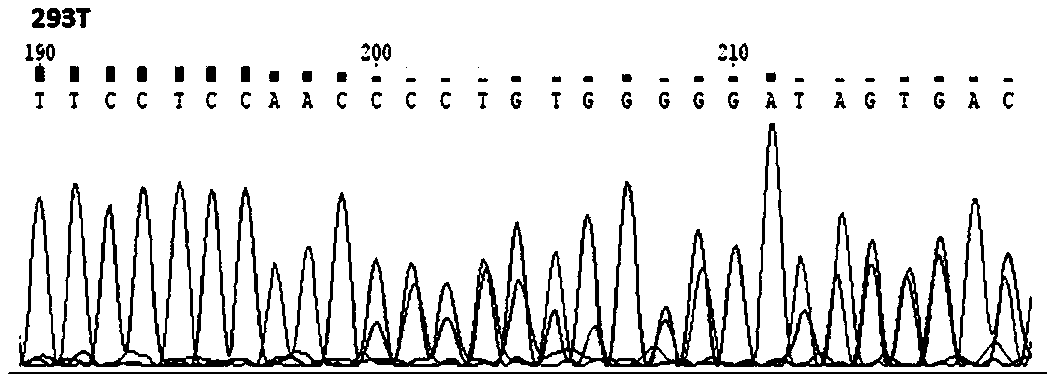
[0013] Example 1 Construction of LMNA gene knockout 293T cell line
[0014] (a) Design of gRNA
[0015] to be knocked out LMNA Based on the complete sequence of the gene, the inventors designed two pairs of gRNA primers, which are:
[0016] gRNA7
[0017] CACCGGCACGCAGCTCCTGGAAGGGT (SEQ ID NO. 1),
[0018] AAACACCCTTCCAGGAGCTGCGTGCC (SEQ ID NO. 2), and
[0019] gRNA8
[0020] CACCGGCGCCGTCATGAGACCCGAC (SEQ ID NO. 3),
[0021] AAACGTCGGGTCTCATGACGGCGCC (SEQ ID NO. 4).
[0022] (b) Vector construction
[0023] (b1) Digestion of empty plasmid and gel purification Digest 1ug of plasmid with restriction endonuclease BbsI, 37°C, 30min:
[0024] BBSI
1ul
PX459
1ug
10xBbSI buffer
5ul
Sterilized water
Make up to 50ul
[0025] Plasmids digested with the QIAquick Gel Extraction Kit were gel purified and eluted in EB.
[0026] (b2) Phosphorylation and annealing gRNA reaction system:
[0027] gRNA-F
1ul
gR...
Embodiment 2
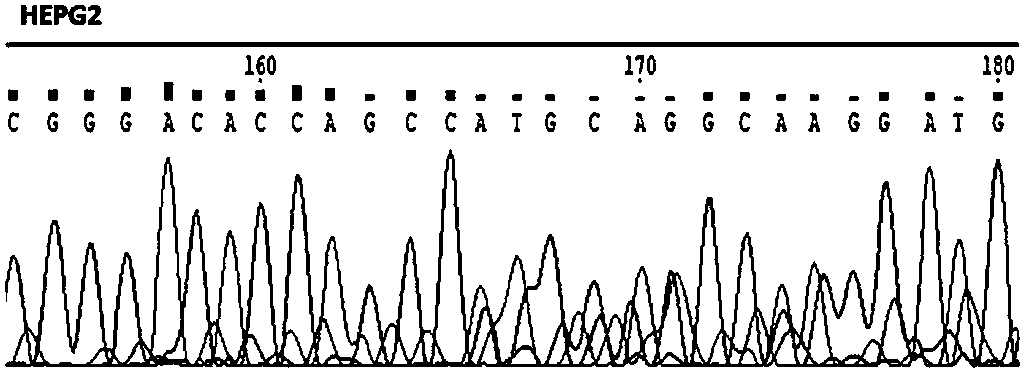
[0052] Construction of the HePG2 cell line of embodiment 2 LMNA gene knockout
[0053] (a) gRNA primer design
[0054] Based on the complete sequence of the lmna gene to be knocked out, the inventors designed two pairs of gRNA primers, which are:
[0055] gRNA 5
[0056] CACCGGTTCCGCCAGCAGCCGCCGGC (SEQ ID NO. 7),
[0057] AAACGCCGGCGGCTGCTGGCGGAACC (SEQ ID NO. 8); and
[0058] gRNA 6
[0059] CACCGGAGCGGGAGATGGCCGAGATG (SEQ ID NO. 9),
[0060] AAACCATCTCGGCCATCTCCCGCTCC (SEQ ID NO. 10).
[0061] (b) Vector construction
[0062] (b1) Digestion of empty plasmid and gel purification Digest 1ug of plasmid with restriction endonuclease BbsI, 37°C, 30min:
[0063] BBSI
1ul
PX459
1ug
10xBbSI buffer
5ul
Sterilized water
Make up to 50ul
[0064] Plasmids digested with the QIAquick Gel Extraction Kit were gel purified and eluted in EB.
[0065] (b2) Phosphorylation and annealing gRNA reaction system:
[0066] gRNA-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com