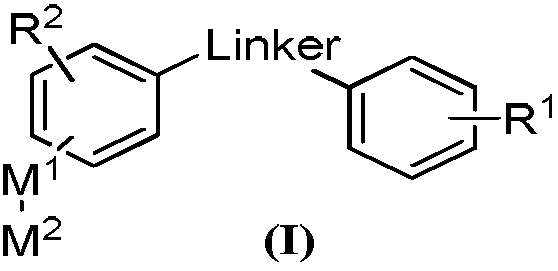
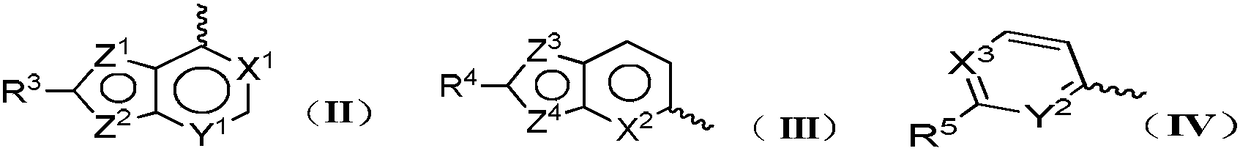
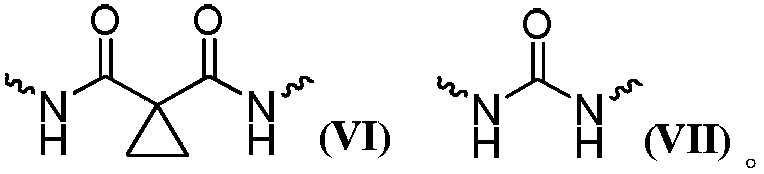Multi-target kinase inhibitor
A protein kinase inhibition, multi-target technology, applied in the field of medicine, can solve the problems of unfavorable liver cancer treatment, toxic side effects, cytotoxicity, etc., and achieve the effect of avoiding the occurrence of toxic side effects and excellent selection specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1 [synthesis of compound]
[0058] (1) Synthesis of compound td32-4
[0059] N-(3-fluoro-4-((2-(1-(2-hydroxy)ethyl)-1H-pyrazol-4-yl)thieno[3,2-b]pyridin-7-yl)oxy Substitute) phenyl)-N-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide (compound td32-4) synthetic route is as follows:
[0060]
[0061] In a 100mL single-necked flask, add compound 5 (2.00g, 5.18mmol, 1.00eq), 1A-4 (1.73 g, 6.22mmol, 1.20eq), Pd(dppf)Cl 2 (379.03mg, 518.00μmol, 0.10eq), Cs 2 CO 3 (5.06g, 15.54mmol, 3.00eq), the above mixture was washed with 60mL THF / H 2 O(5:1, v:v) was dissolved, and the resulting solution was stirred and reacted at 50° C. for 5 hours under nitrogen protection. The reaction was monitored by LC-MS until the complete disappearance of compound 5 and the formation of the target product 1-4. After the reaction was completed, 50 mL of purified water was added, extracted with ethyl acetate three times (50 mL each time), and the obtained organic layer was rotary e...
Embodiment 2
[0189] Example 2【Screening of Compounds for Inhibitory Activity of Protein Kinase】
[0190] Using the methods of Mobility Shift Assay and Lanthascreen Assay, the inhibitory activities of representative compounds (shown in Table 1) on the following kinases were tested: VEGFR2, c-Met, c-kit, B-Raf, EGFR, RET.
[0191] Table 1 Purchase information of the kinases used
[0192] Kinase name
source
product number
BRAF
Invitrogen
PR 6995A
1258788L
CKIT
Millipore
14-559k
2060980
EGFR
Carna
08-115
13CBS-0005L
VEGFR2
Carna
08-191
07CBS-0540
CMET
Carna
08-151
10CBS-1118K
RET
Carna
08-159
06CBS-3284
[0193] The experimental method is as follows:
[0194] 1) Prepare 1x kinase buffer and stop solution
[0195] 1x kinase buffer: 50mM HEPES, pH 7.5, 0.0015% Brij-35, 2mM DTT;
[0196] Stop solution: 100 mM HEPES, pH 7.5, 0.015% Brij-35.
[...
Embodiment 3
[0214] Example 3 [Test of Compound Inhibiting Tumor Cell Proliferation Activity in Vitro]
[0215] According to the results of protein kinase inhibitory activity in Example 2, the compounds whose effect is better than or similar to that of the reference products Sorafenib and Cabozantinib were selected, that is, compounds td32-4, td32-5, td32-6, t-3, 51, and 29 were selected for Inhibitory cell proliferation activity test.
[0216] Instruments and materials:
[0217] Cell Titer-Glo luminescent cell viability assay (Promega, Cat. No. G7573, Lot. No. 0000181739).
[0218] TT (ATCC, Cat.No.CRL-1803, Lot.No.58785858)
[0219] SNU-5 (ATCC, Cat.No.CRL-5973, Lot.No.58033358)
[0220] Hs746T (ATCC, Cat.No.HTB-135, Lot.No.5006453)
[0221] U87MG (ATCC, Cat.No.HTB-14, Lot.No.5018014)
[0222] HepG2 (ATCC, Cat. No. HB-8065, Lot. No. 7579337)
[0223] A673 (ATCC, Cat.No.CRL-1598, Lot.No.58075870)
[0224] F-12K medium (Invitrogen, Cat.No.21127-022, Lot.No.1759876)
[0225] MEM med...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com