Patents
Literature
44 results about "Inhibitory cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The inhibitory nerve cells we seek to produce will reduce brain activity in target regions. They may therefore be used to treat other conditions characterized by excessive brain activity, such as epilepsy. Epilepsy can be a life threatening and disabling condition.
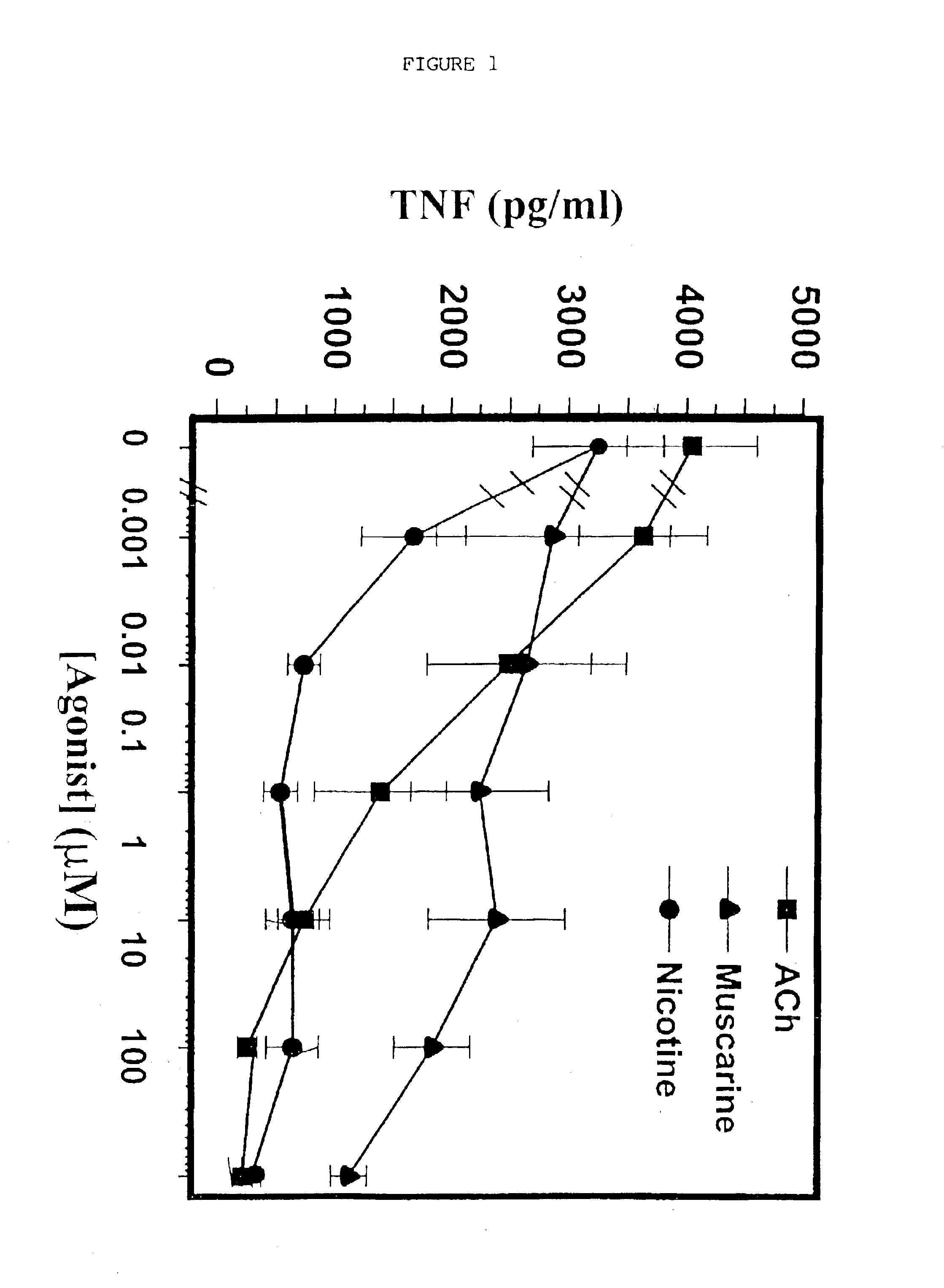
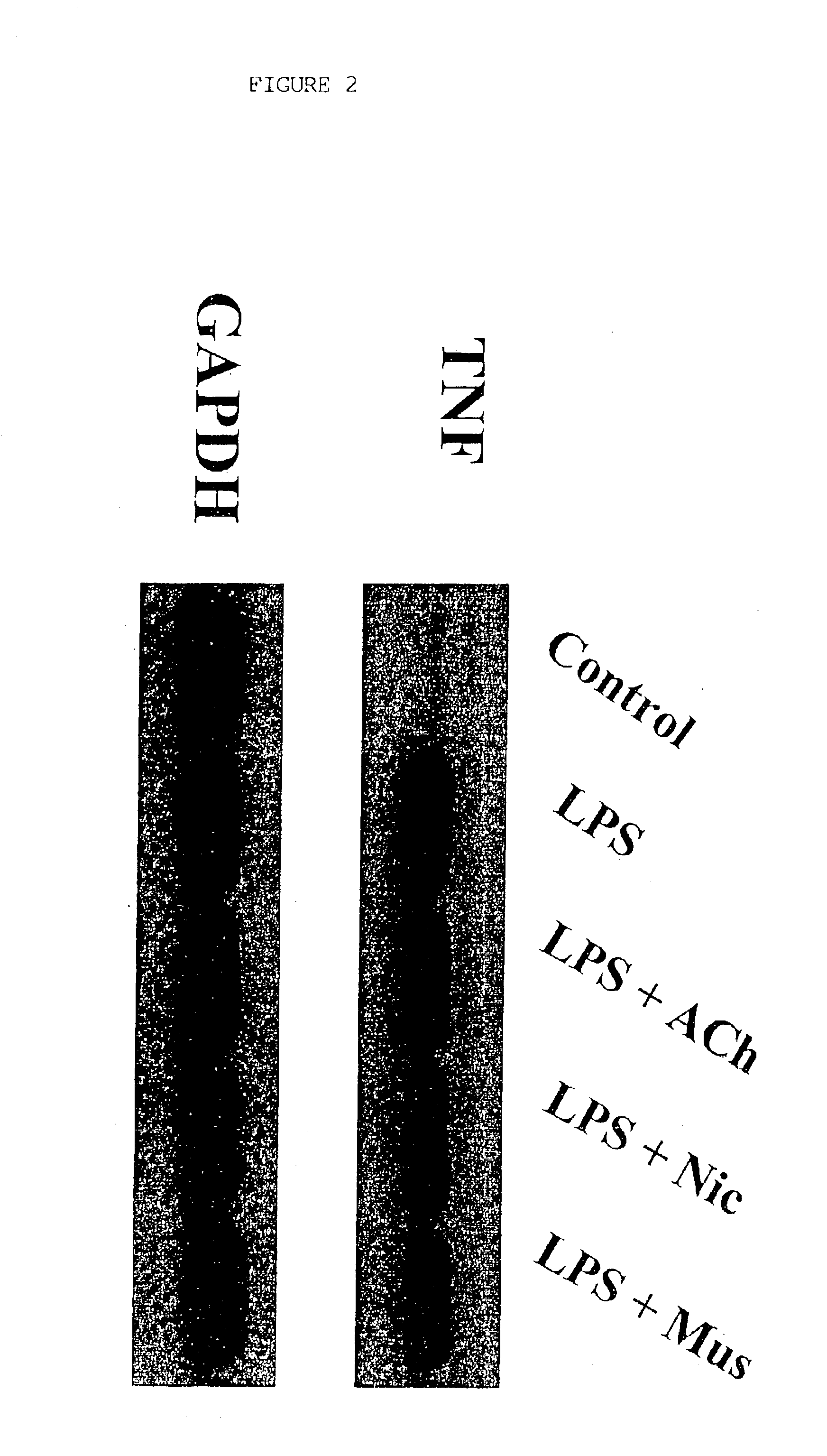
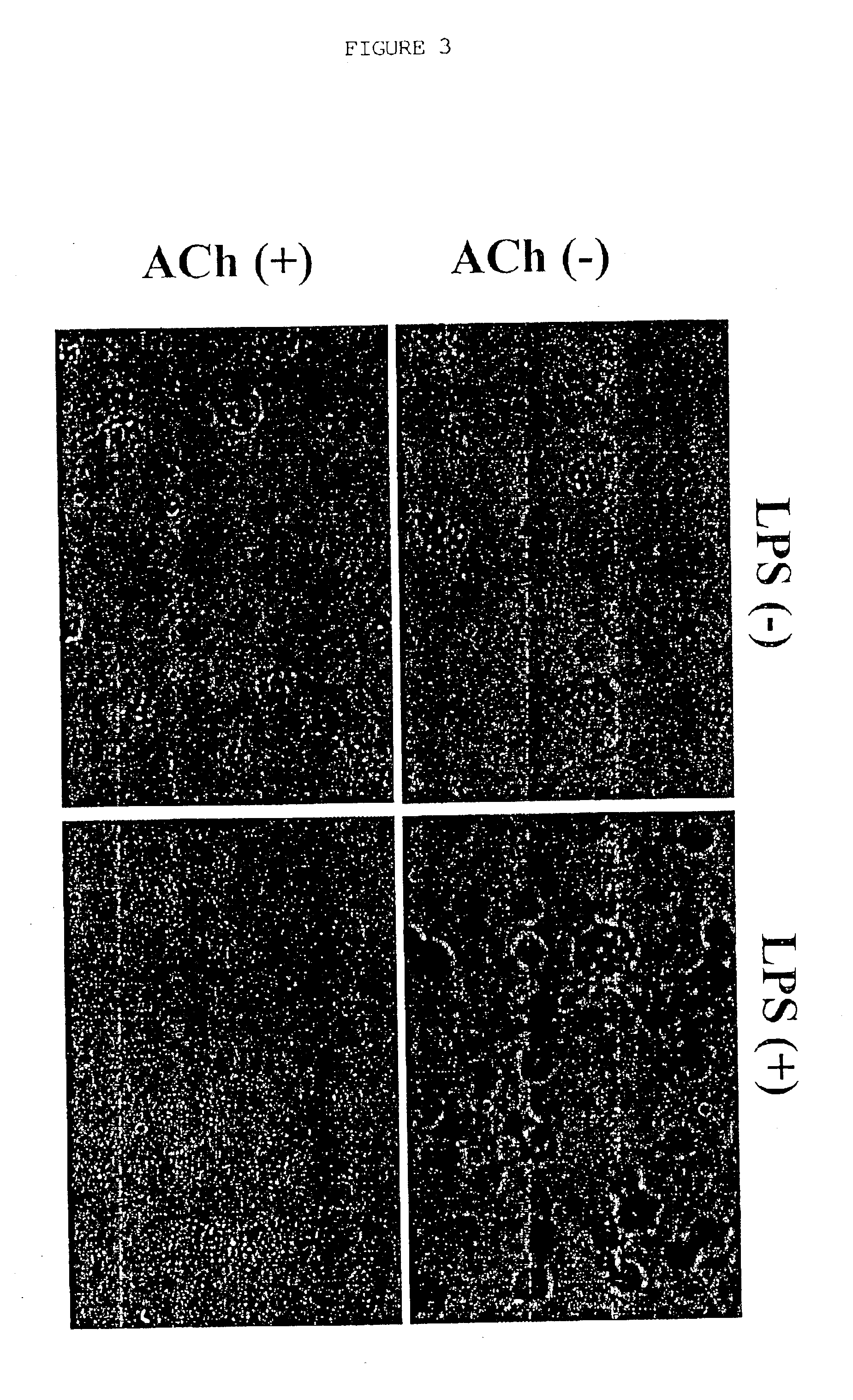
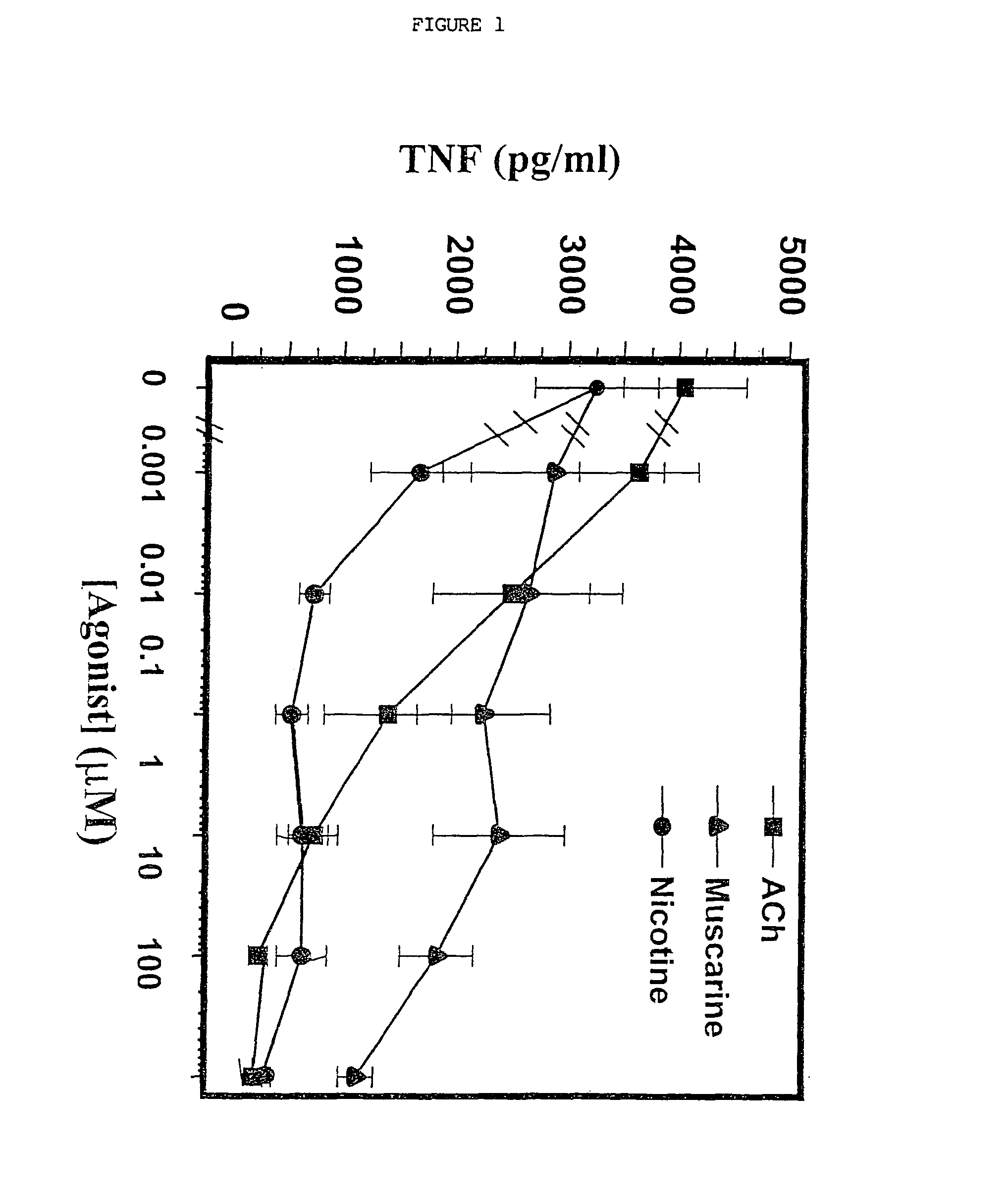
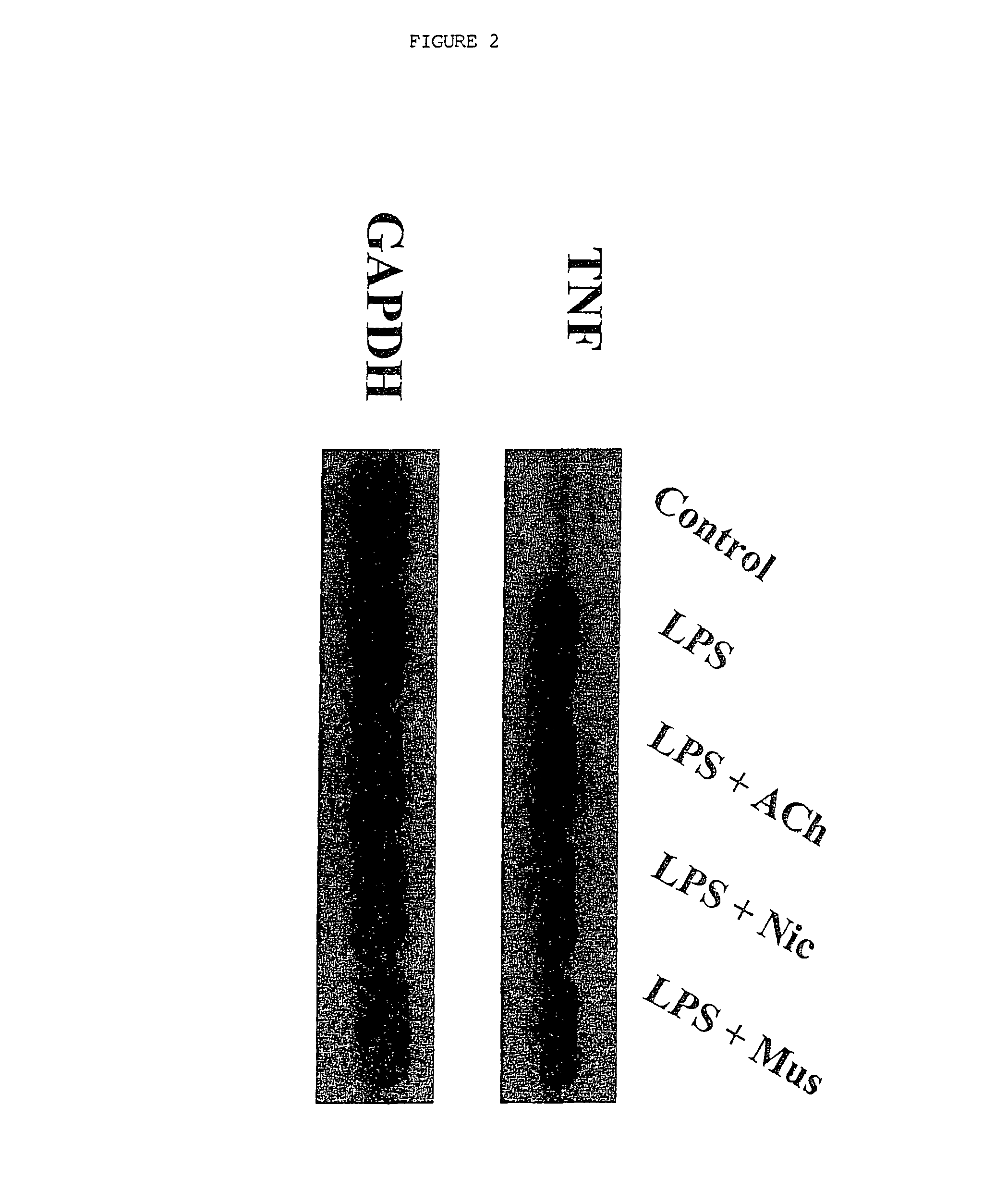
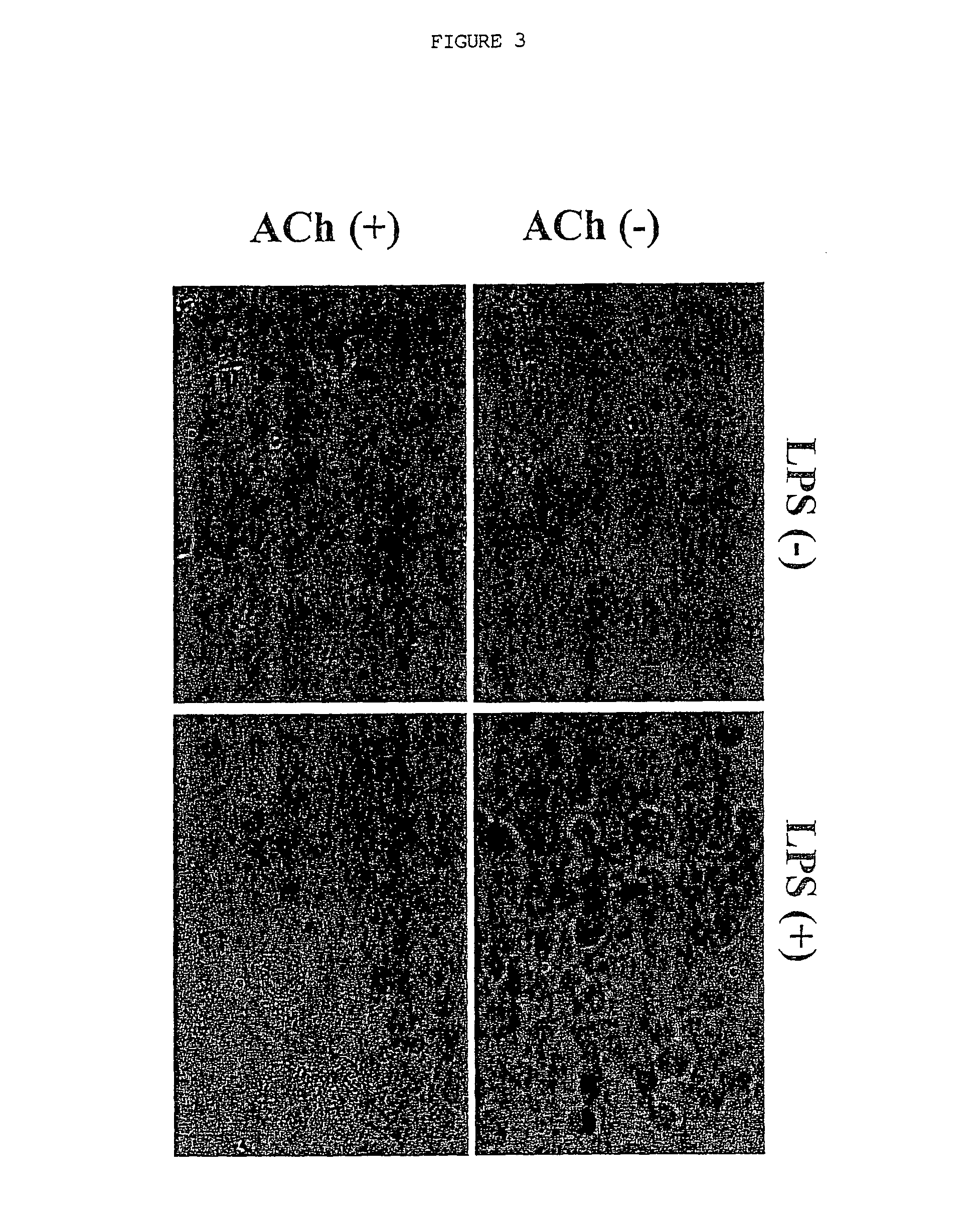
Inhibition of inflammatory cytokine production by cholinergic agonists and vagus nerve stimulation
A method of inhibiting the release of a proinflammatory cytokine in a cell is disclosed. The method comprises treating the cell with a cholinergic agonist. The method is useful in patients at risk for, or suffering from, a condition mediated by an inflammatory cytokine cascade, for example endotoxic shock. The cholinergic agonist treatment can be effected by stimulation of an efferent vagus nerve fiber, or the entire vagus nerve.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Inhibition of inflammatory cytokine production by cholinergic agonists and vagus nerve stimulation
InactiveUS8914114B2Implantable neurostimulatorsHeterocyclic compound active ingredientsSacral nerve stimulationEfferent
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Inhibition of STAT3 signal transduction for human cancer therapy
InactiveUS20070060521A1Improve efficiencyIncrease in intracellular expression of Bcl-xOrganic active ingredientsFungiAbnormal tissue growthHuman cancer
Signal Transducer and Activator of Transcription (STAT) proteins have a fundamental role cell signaling, and are activated by a large number of cytokines and growth factors. One member of the STAT family, STAT3, has a critical role in oncogenesis. The present invention relates generally to disruption of the pathway of STAT3 signaling in the treatment of human cancer. STAT3 activation is shown to be present in diverse tumor cell lines and tumors, to promote oncogenesis, to inhibit apoptosis, and to reduce sensitivity to chemotherapeutic agents. Inhibition of STAT3 signaling induces apoptosis specifically in tumor cell lines, and increases sensitivity to chemotherapeutic agents. The invention relates more particularly to methods, compositions, means of administering such compositions, and means for identifying such compositions for the inhibition of STAT3 intracellular signaling in the treatment of human cancers.
Owner:YALE UNIV +1
Methods and compositions for treating secondary tissue damage and other inflammatory conditions and disorders
InactiveUS7157418B1Enhance and aid in survivalPrevent proliferationAntibacterial agentsBiocidePhagocyteDendritic cell
Conjugates containing as a ligand a chemokine receptor targeting agents, such as chemokines, and a targeted agent, such as a toxin are provided. These conjugates are used to treat inflammatory responses associated with activation, proliferation and migration of immune effector cells, including leukocyte cell types, neutrophiles, macrophages, and eosinophils. The conjugates provided herein are used to lessen or inhibit these processes to prevent or at least lessen the resulting secondary effects. In particular, the conjugates are used to target toxins to receptors on secondary tissue damage-promoting cells. The ligand moiety can be selected to deliver the cell toxin to such secondary tissue damage-promoting cells as mononuclear phagocytes, leukocytes, natural killer cells, dendritic cells, and T and B lymphocytes, thereby suppressing the proliferation, migration, or physiological activity of such cells. Among preferred conjugates are fusion proteins having a chemokine, or a biologically active fragment thereof, as the ligand moiety linked to a cell toxin via a peptide linker of from 2 to about 60 amino acid residues.
Owner:OSPREY PHARMA USA INC
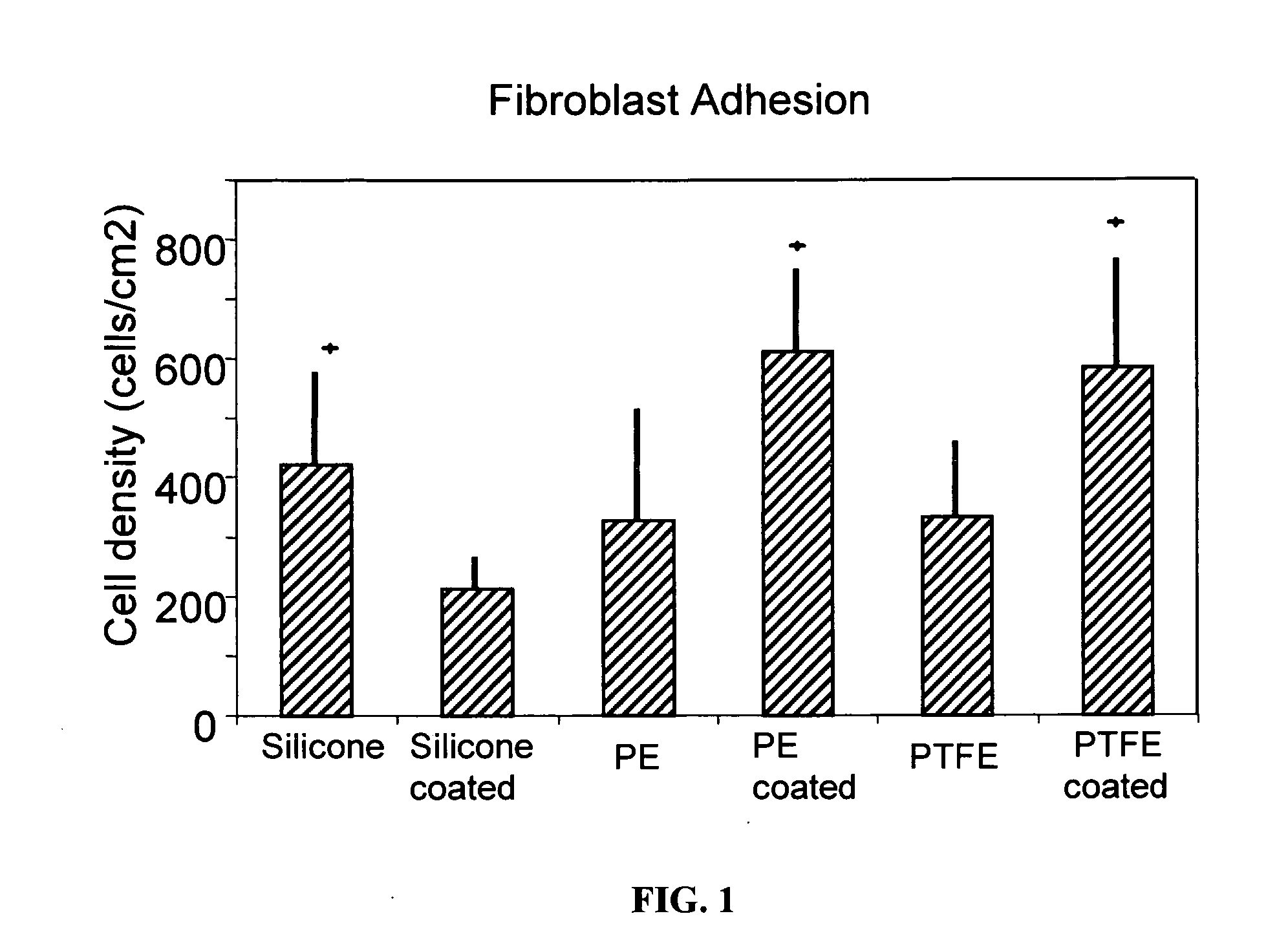
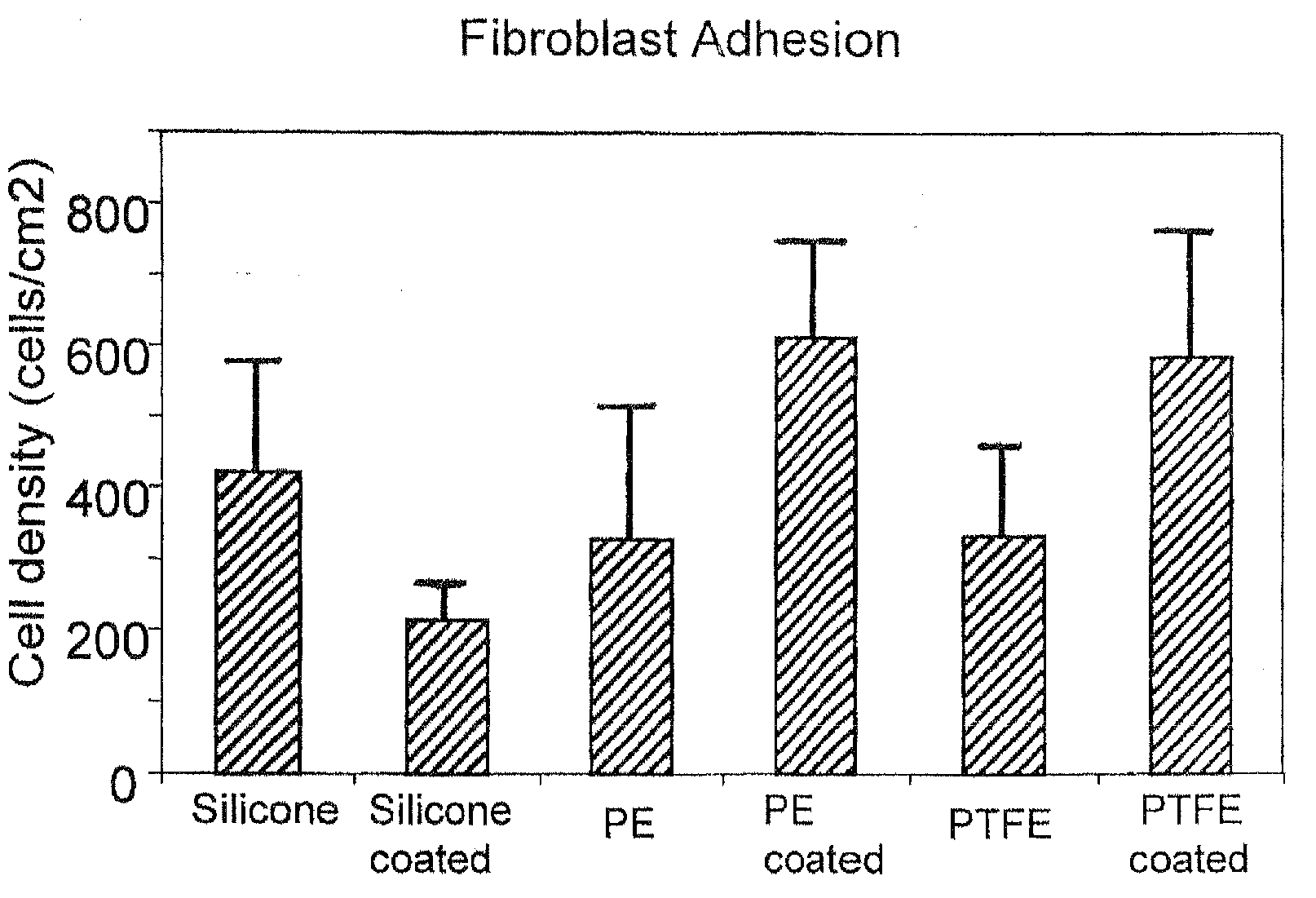
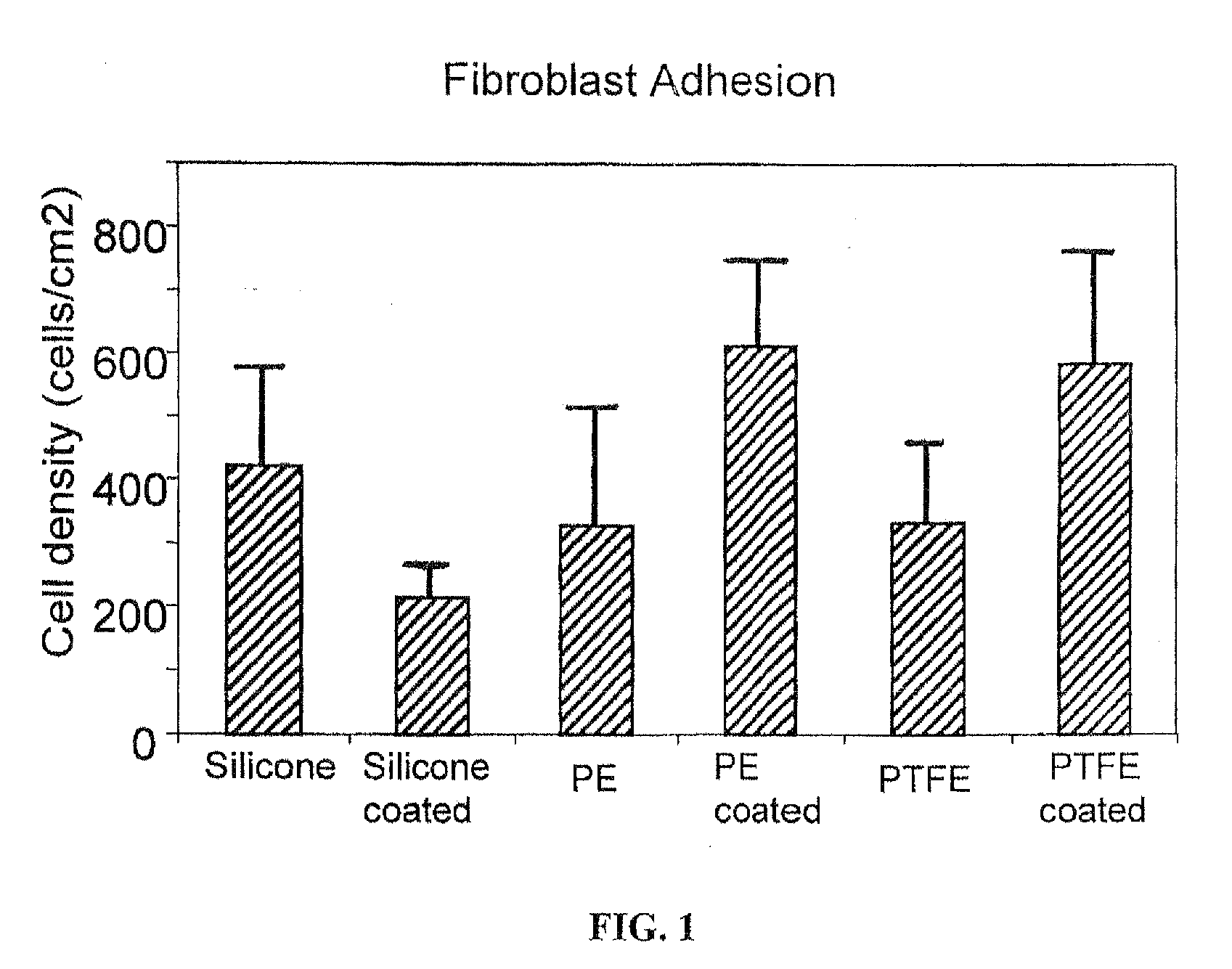
Inhibitory cell adhesion surfaces
InactiveUS20080275546A1Efficient changeHigh surface energyStentsMammary implantsCell adhesionIn vivo
Textured nanostructured surfaces are described which are highly resistant to cell adhesion. Such surfaces on medical implants inhibit fibroblast adhesion particularly on titanium treated silicone. The surfaces can also be engineered so that other cell types, such as endothelial and osteoblast cells, show little if any tendency to attach to the surface in vivo.
Owner:NANOSURFACE TECH
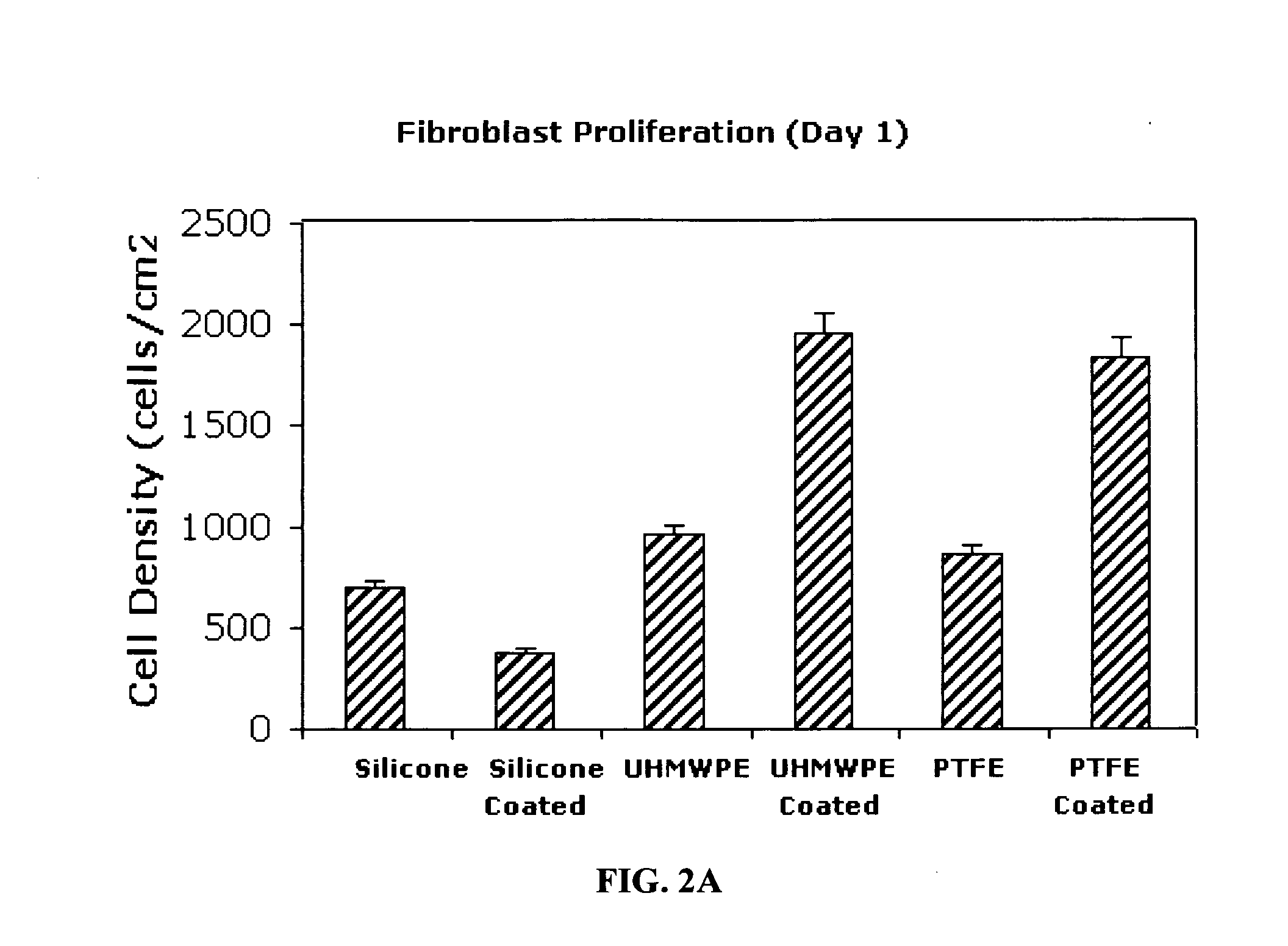
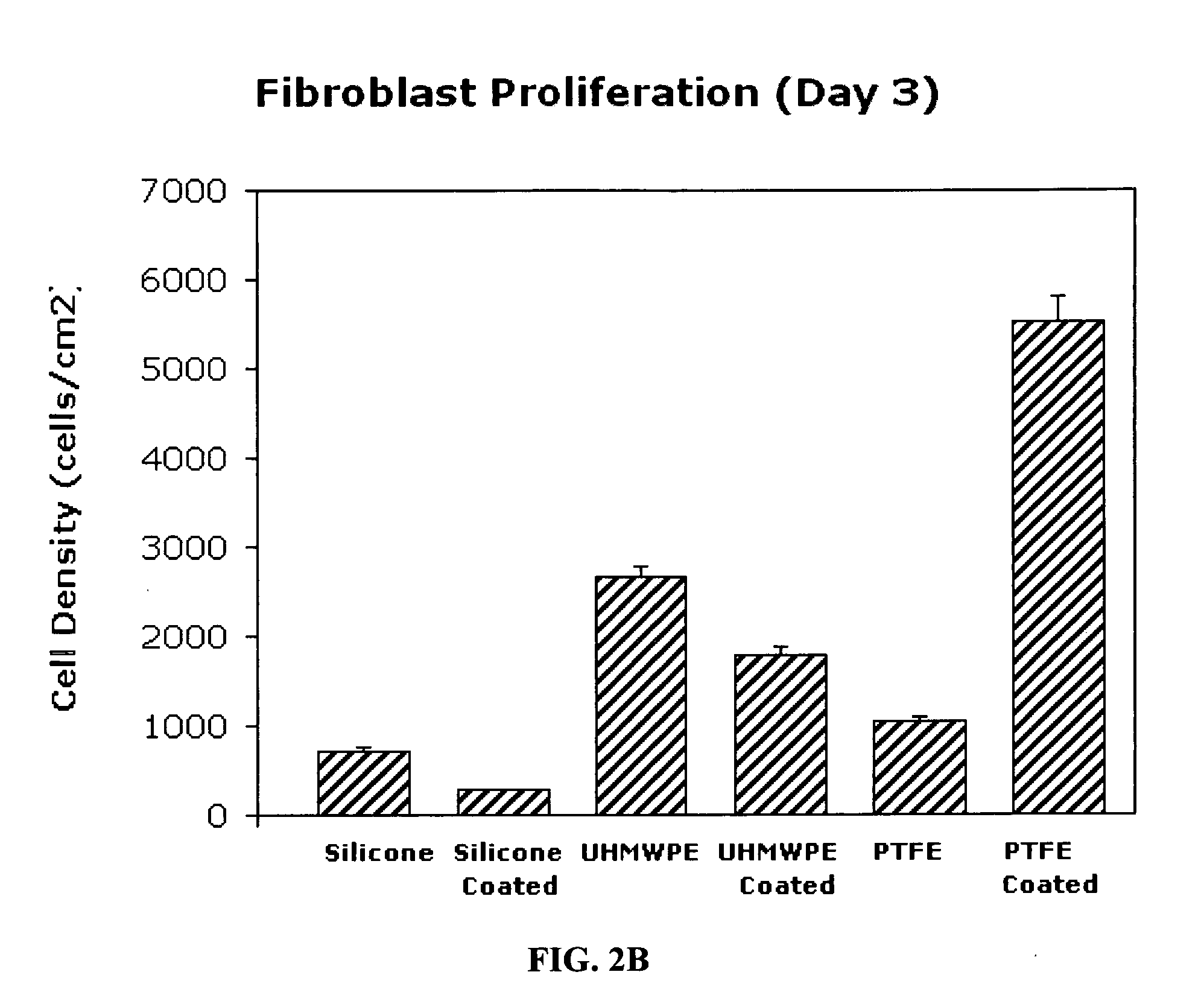
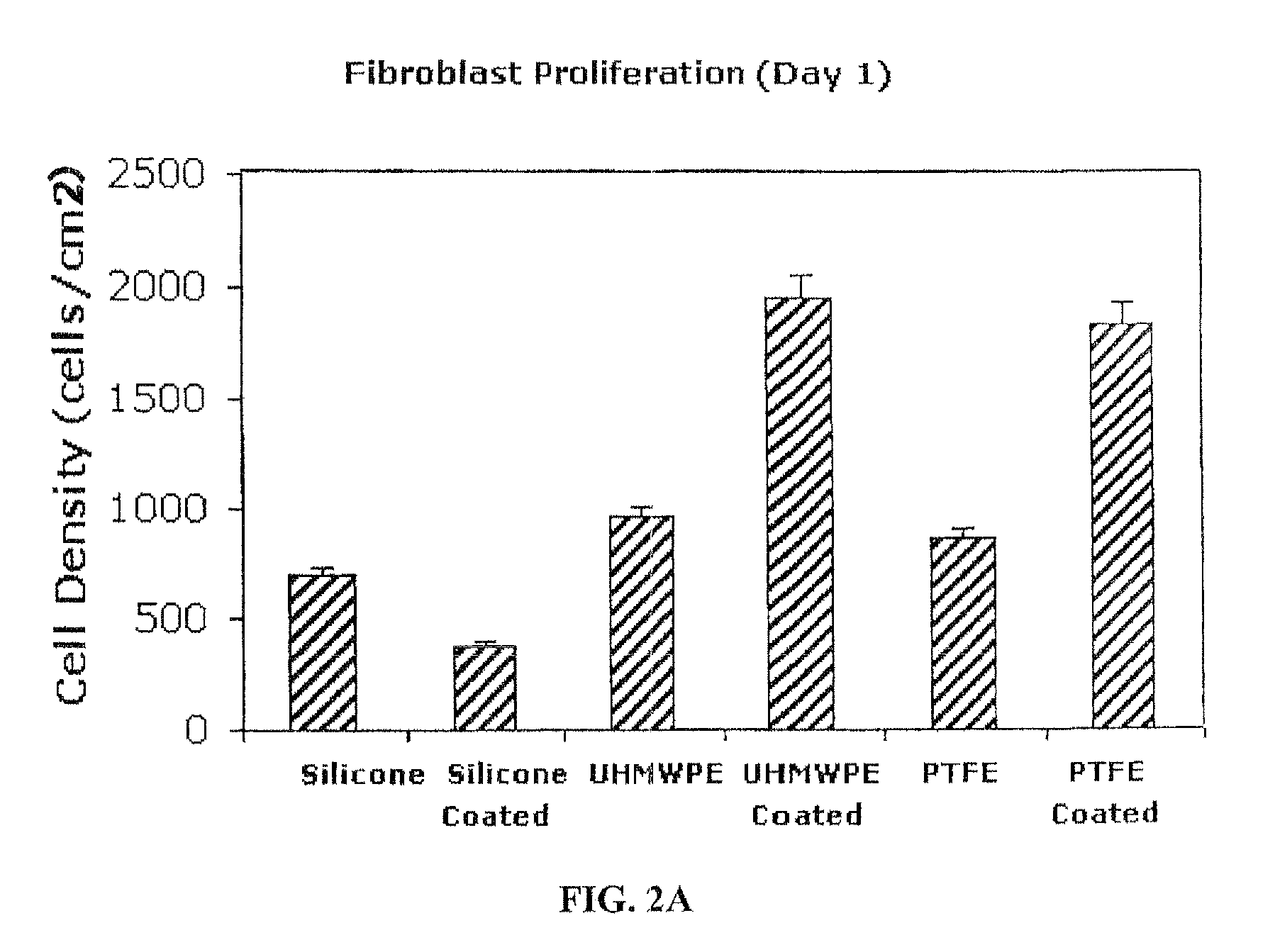
Inhibitory cell adhesion surfaces
Nanostructured surfaces on selected substrates are described which are highly resistant to cell adhesion. Such surfaces on medical implants inhibit fibroblast adhesion particularly on nanorough titanium deposited on smooth silicone surfaces. The nanostructured deposited metal coatings can also be engineered so that several cell types, including endothelial, osteoblast, and fibroblast cells, show little if any tendency to attach to the coated surface in vivo.
Owner:METASCAPE
Method and screening candidate compounds for drug against tumor
InactiveUS7125659B1Eliminate the effects ofBiocideMicrobiological testing/measurementAbnormal tissue growthPhosphorylation
Investigation on the frequency of FLT3 / ITD found in various blood cancers has revealed that the frequency is high in acute myeloblastic leukemia in particular. Studies on the effects of FLT3 / ITD in the blood cell lines revealed that the tyrosine residues in FLT3 / ITD is constitutively phosphorylated in these cell lines and that blood cells into which FLT3 / ITD is introduced show IL-3 independent proliferation. Moreover, the blood cells into which FLT3 / ITD is introduced are found to be capable of forming tumors and inhibit cell differentiation. The inventors have found that it is possible to screen for pharmaceutical compounds against tumors by using inhibition of these FLT3 / ITD functions as an index.
Owner:CHUGAI PHARMA CO LTD
Mechanically activated channel blocker
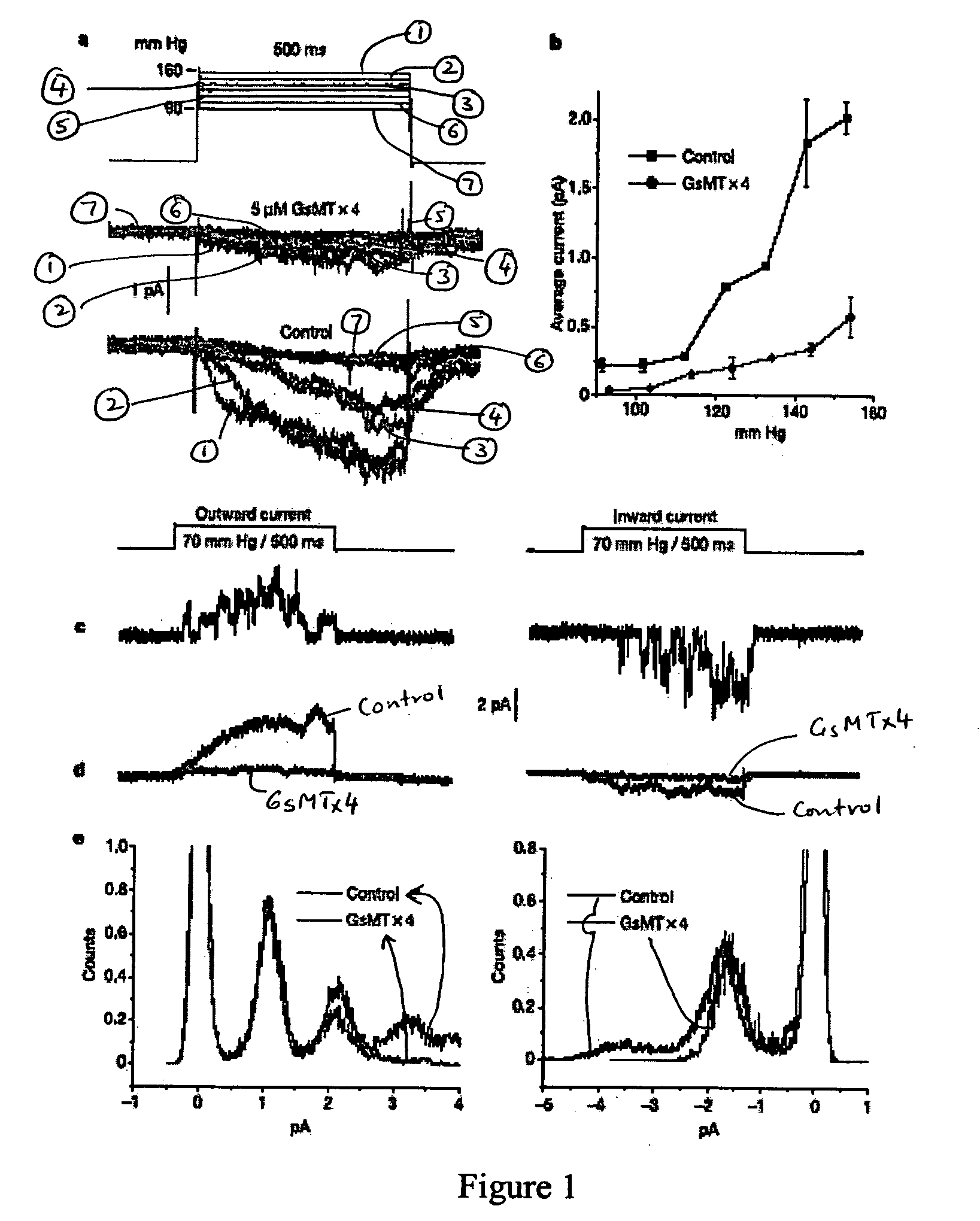
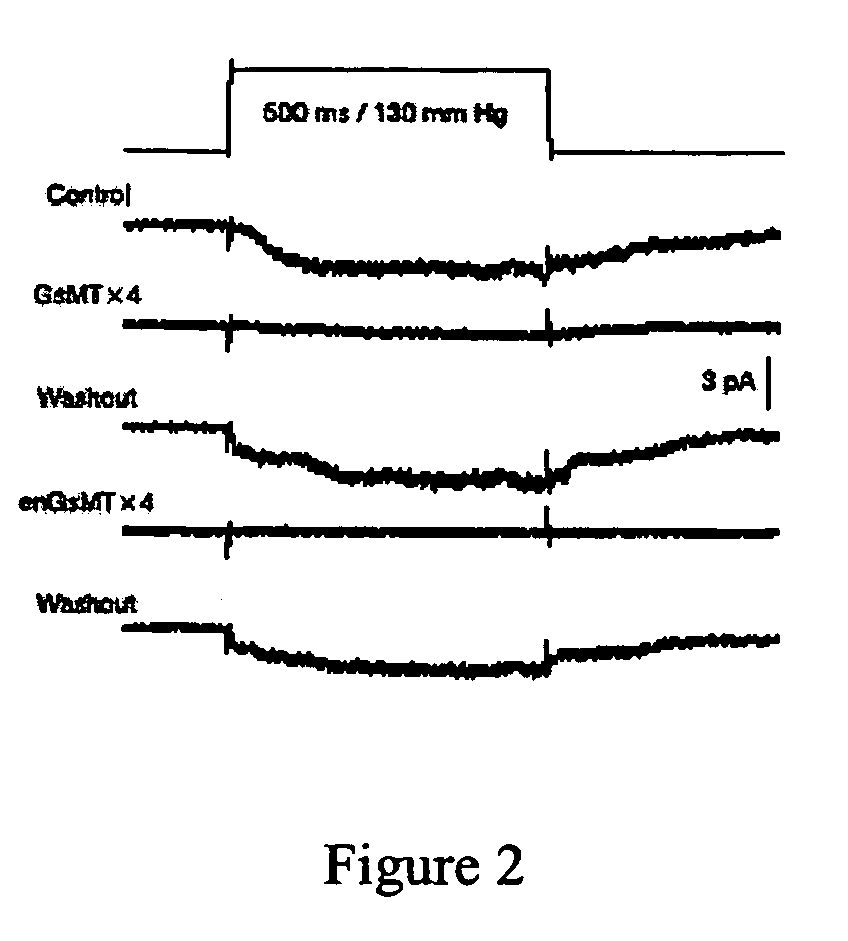
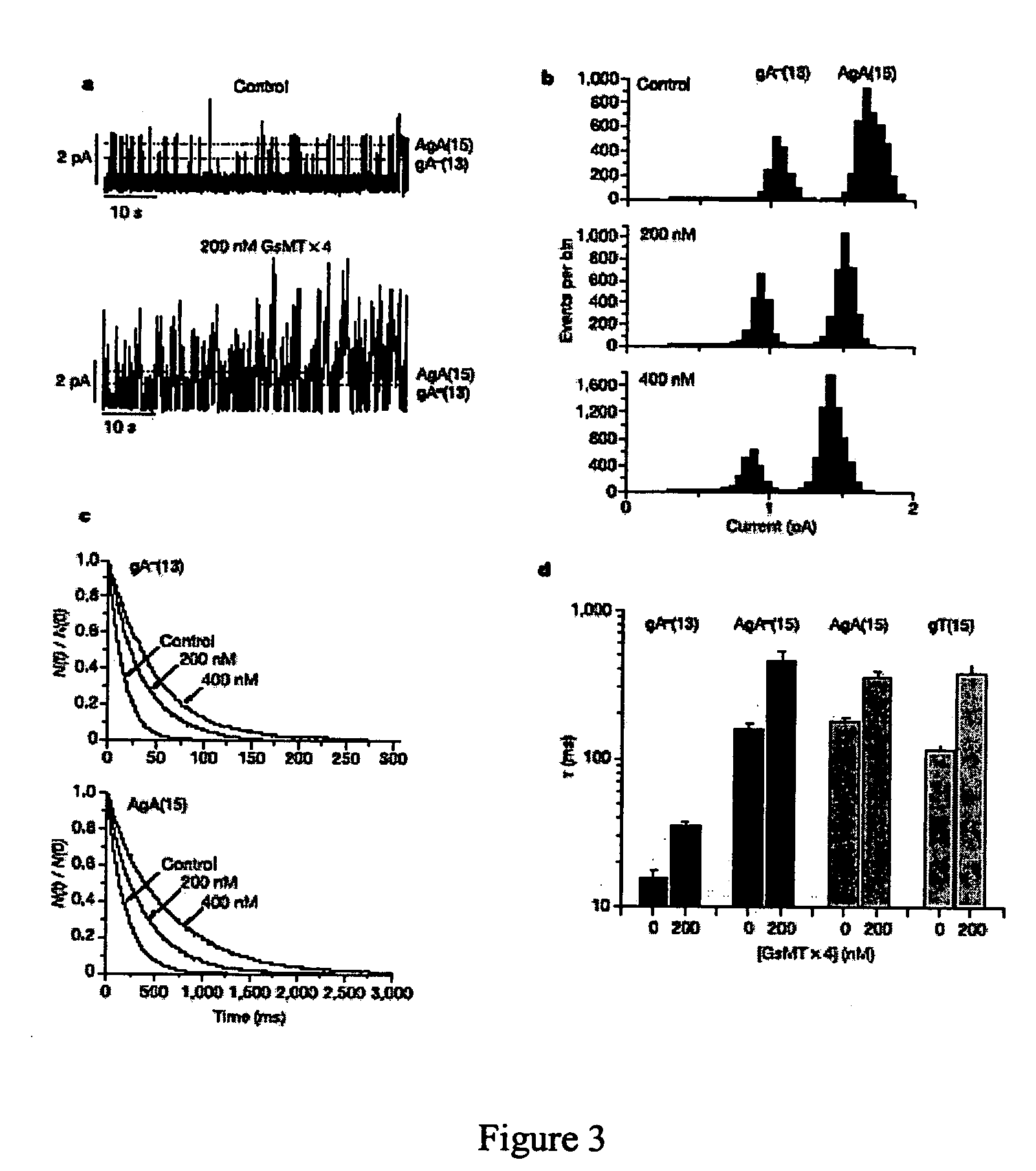
The present invention discloses a peptide of SEQ ID NO:1 and its variants that blocks stretch-activated ion channels. All amino acids in this peptide are D-amino acids. The peptide, designated as D-GsMTx4, is an enantiomer of a peptide GsMTX-4 present in the venom of the spider Grammostola spatulata. The present invention also discloses a method for inhibition of stretch activated ion channels in a cell. This peptide can be used for the treatment of cardiac arrhythmias and other pathologies that involve alteartions in mechanical stress.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
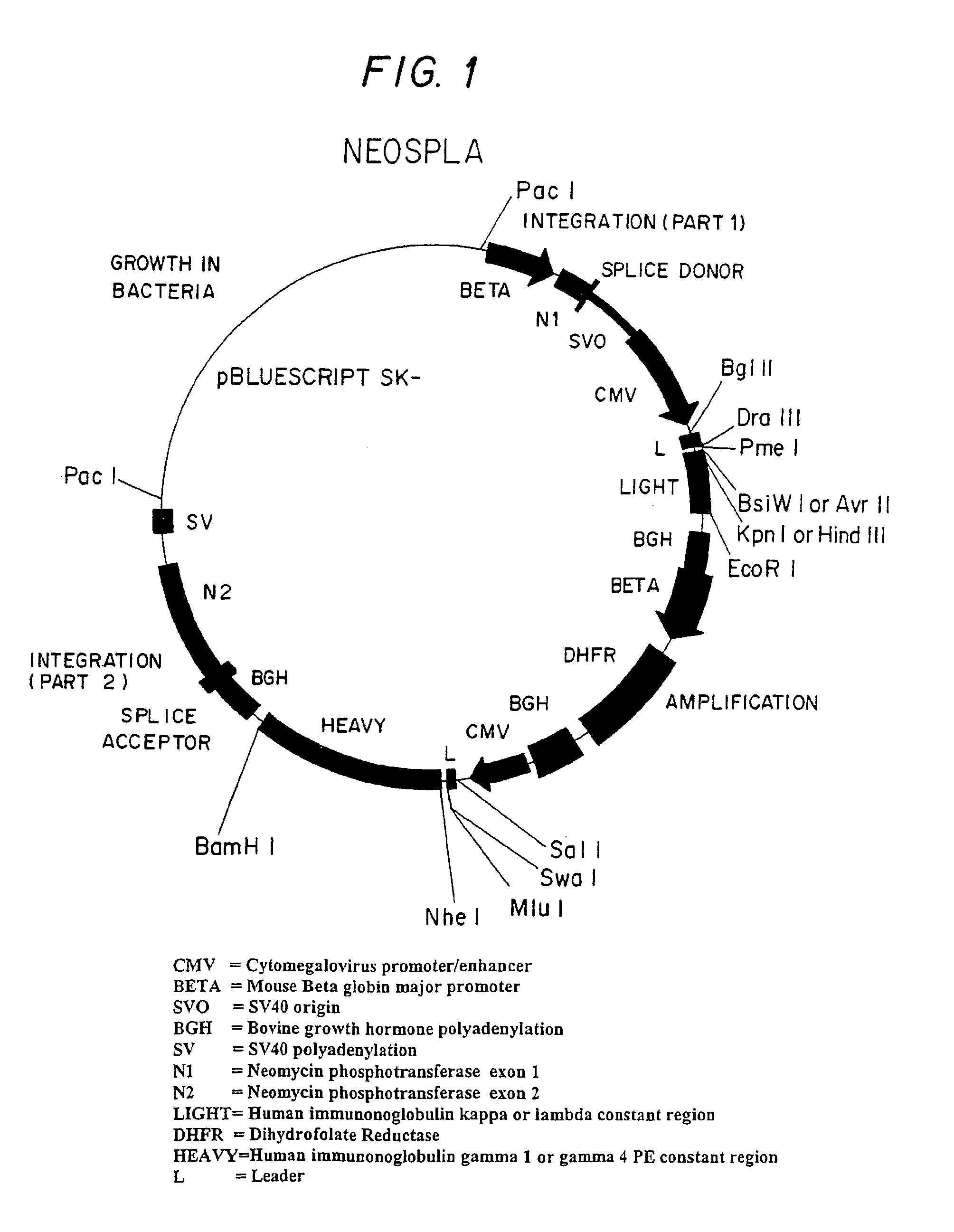
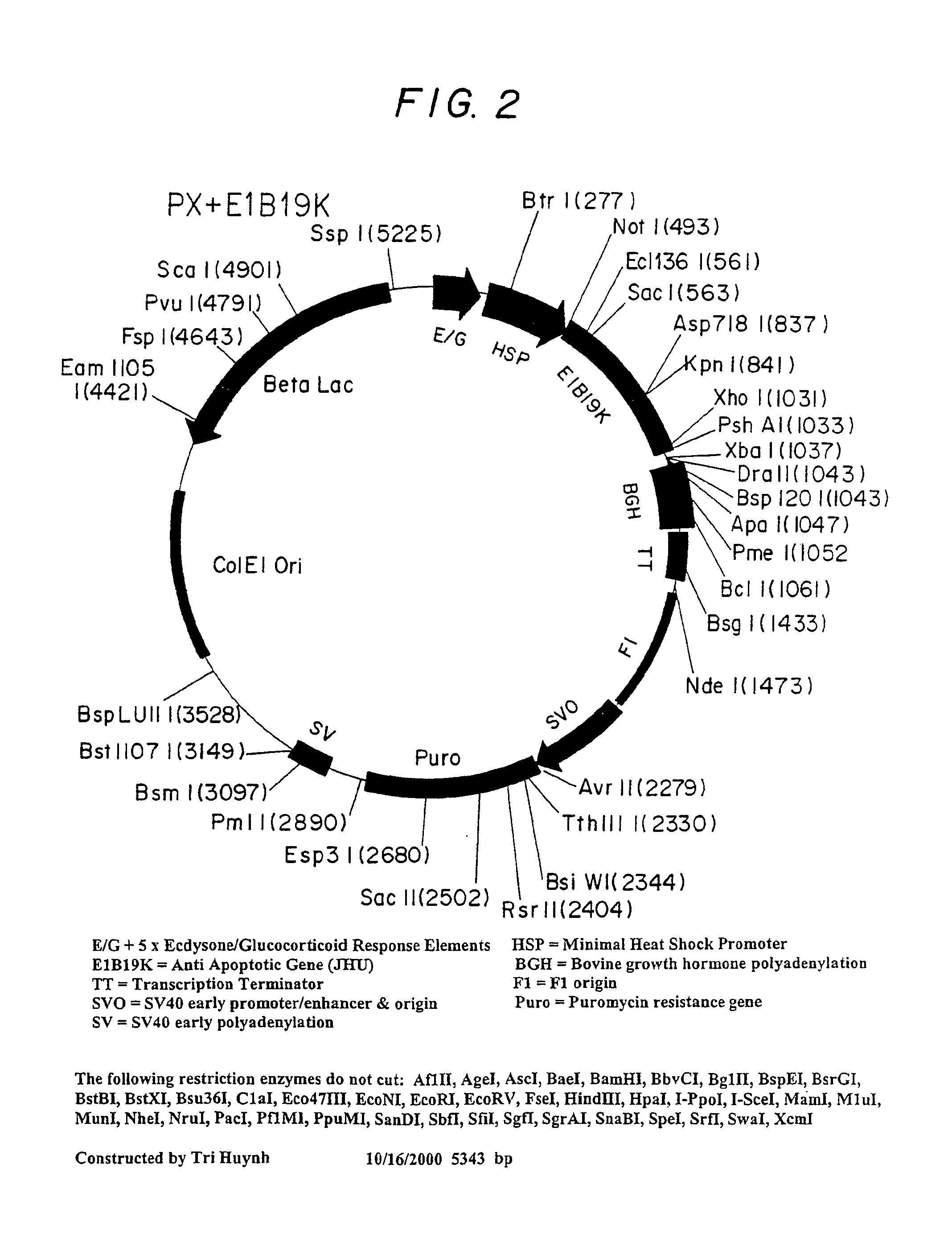
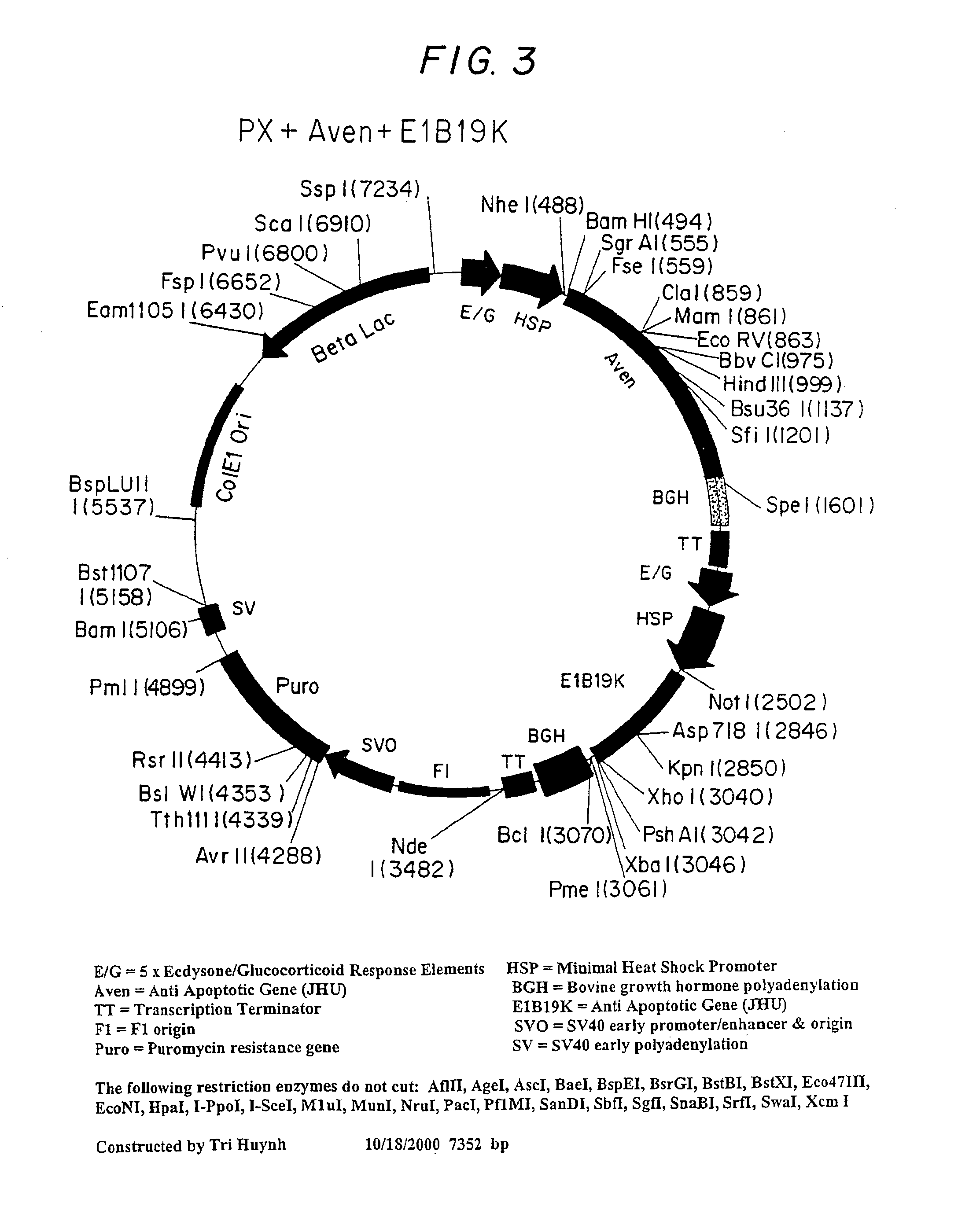
Inhibition of apoptosis process and improvement of cell performance
The present invention relates to preventing or delaying programmed cell death by expressing one or more anti-apoptotic polypeptides in a cell such that programmed cell death in the cell is prevented or delayed. The present invention also relates to increasing production of a cell-related product by expressing one or more anti-apoptotic polypeptides in a cell such that production of the cell-related product by the cell is increased. Recombinant cells useful for producing cell-related product or cellular therapy are also provided.
Owner:BIOGEN INC
Application of circRNA in field of angiogenesis inhibition
ActiveCN109432119AChange structureInhibition formationOrganic active ingredientsSenses disorderDiseaseVascular endothelium
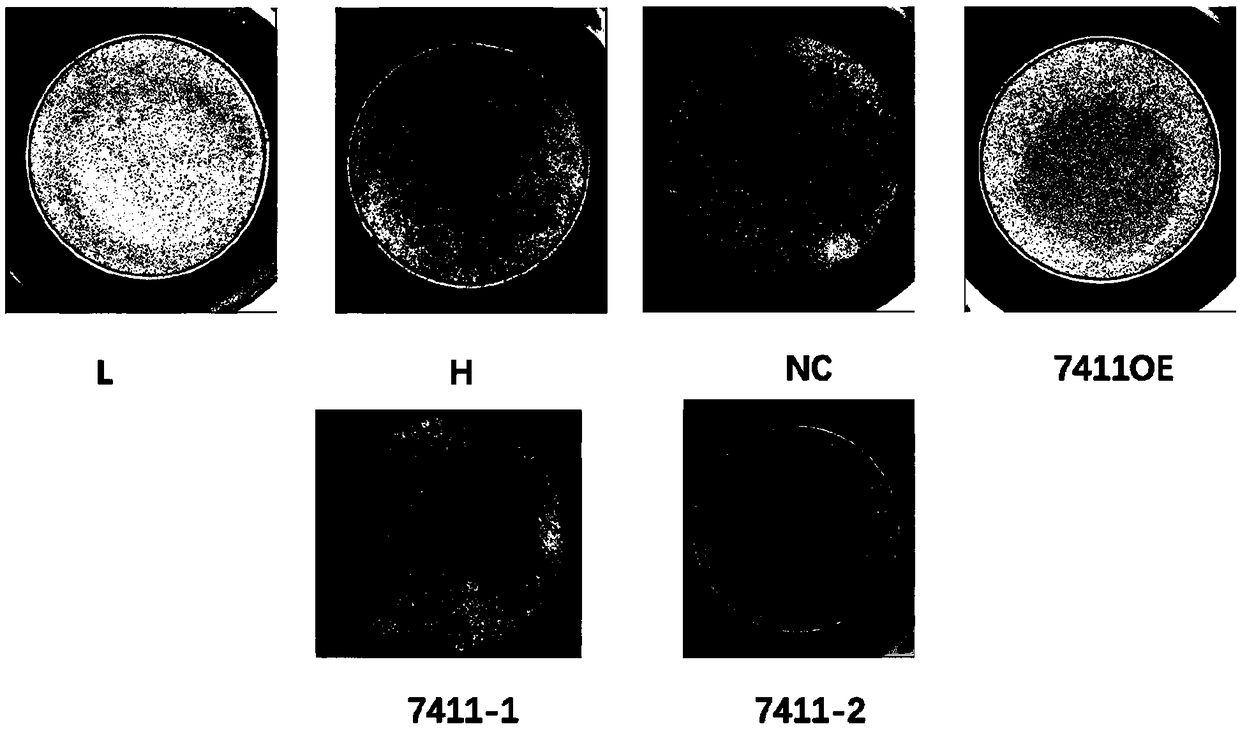
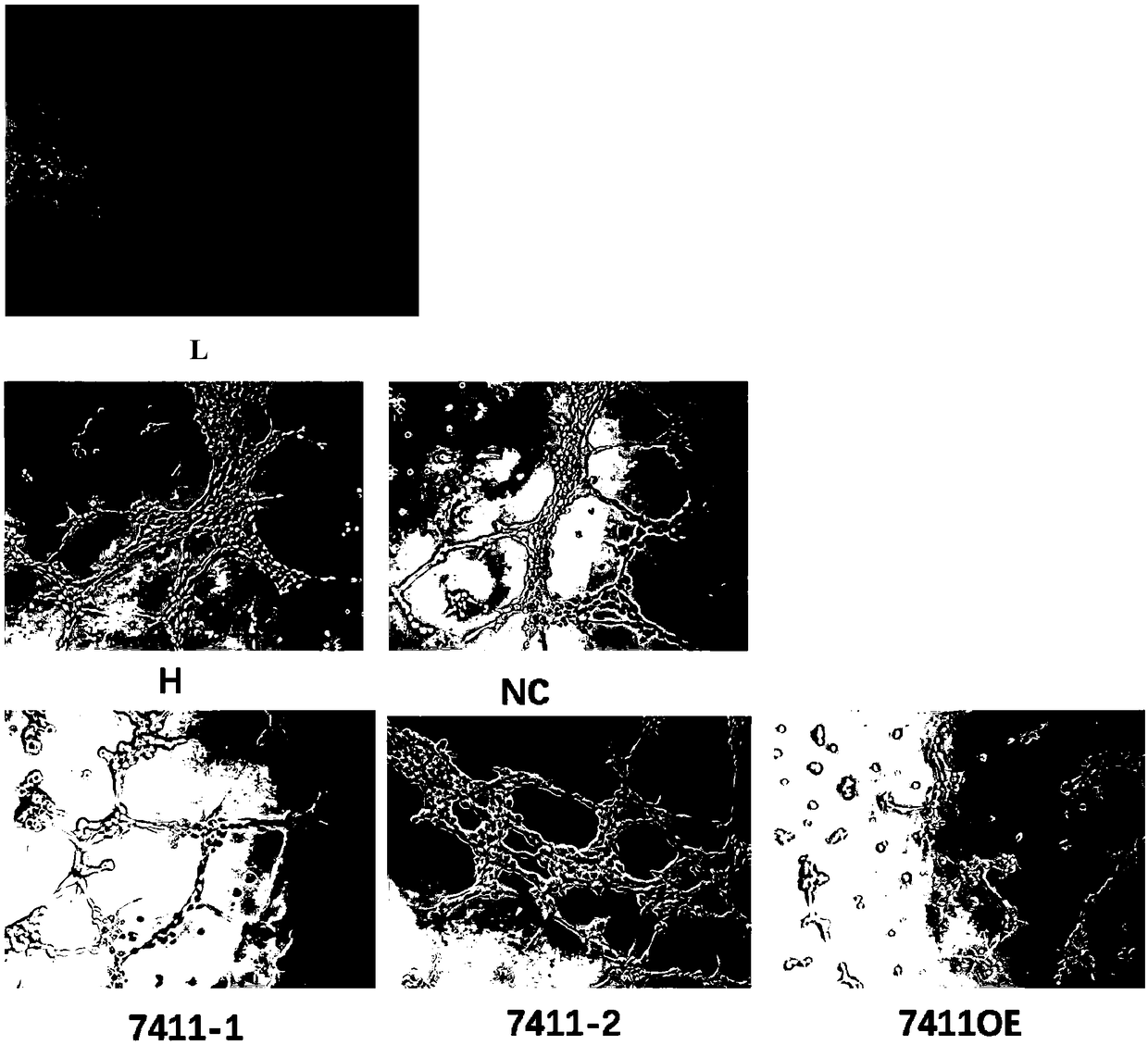
The invention discloses application of circRNA in the field of angiogenesis inhibition, and belongs to the fields of biochemistry, molecular biology and medicine. The invention discovers that hsa_circ_0007411 can continuously act on eye vascular endothelial cells, change the structure of angiogenesis and inhibit angiogenesis. By applying the hsa_circ_0007411 to the eye microvascular endothelial cells, 90 percent of angiogenesis can be effectively inhibited, and angiogenesis of neovascularization-related diseases can be effectively inhibited. The effect of hsa_circ_0007411 on cell proliferationand migration is verified, thereby proving that hsa_circ_0007411 can remarkably inhibit cell proliferation and migration, has a remarkable inhibition effect on the proliferation of endothelial cells,and has important guiding significance for diseases related to the proliferation of vascular endothelial cells.
Owner:易舟(上海)生物医药有限公司
Myeloid derived suppressor cell inhibiting agents
ActiveUS9320735B2Improve immune activityImprove efficiencySnake antigen ingredientsAntibody ingredientsAdjuvantCancer metastasis
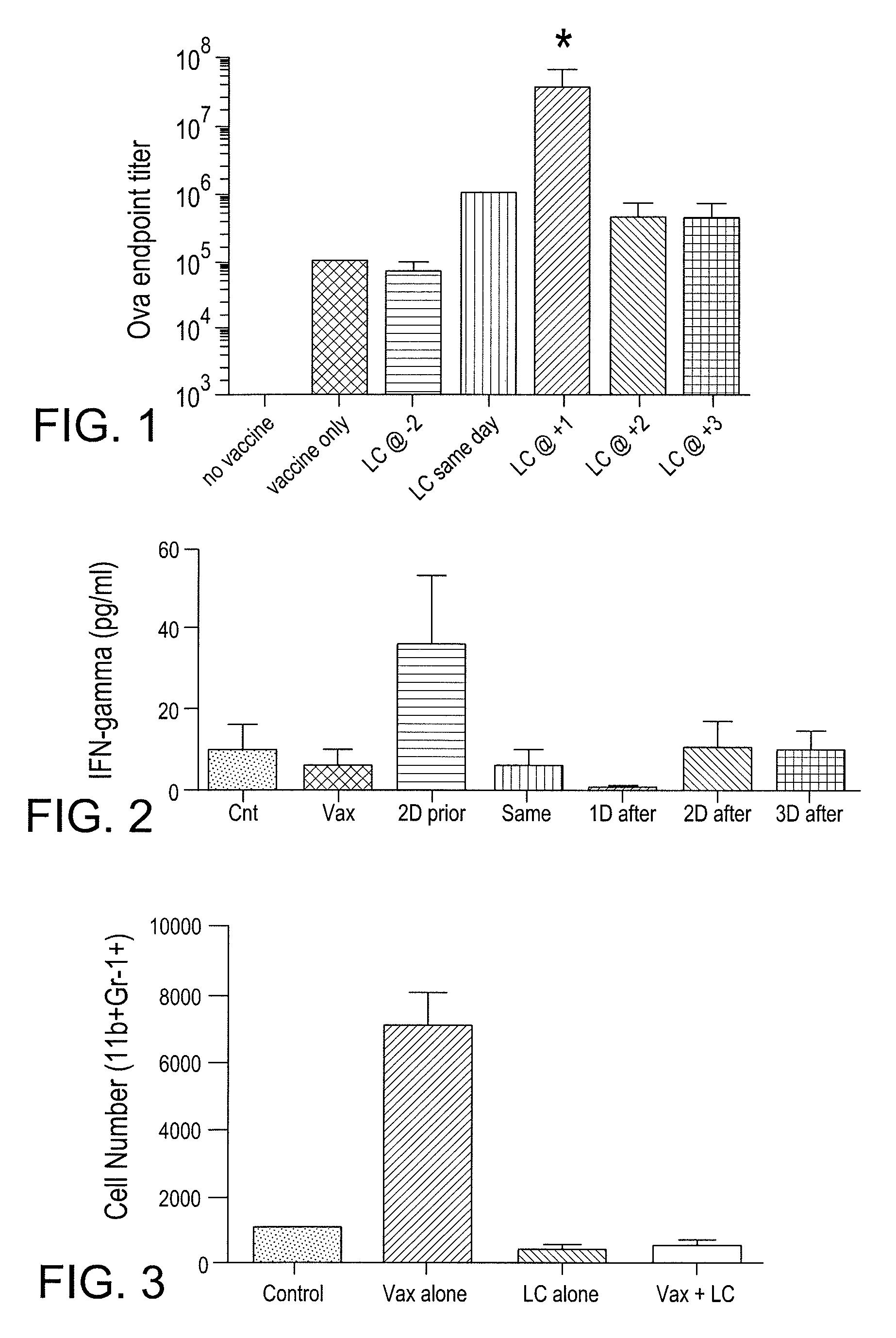
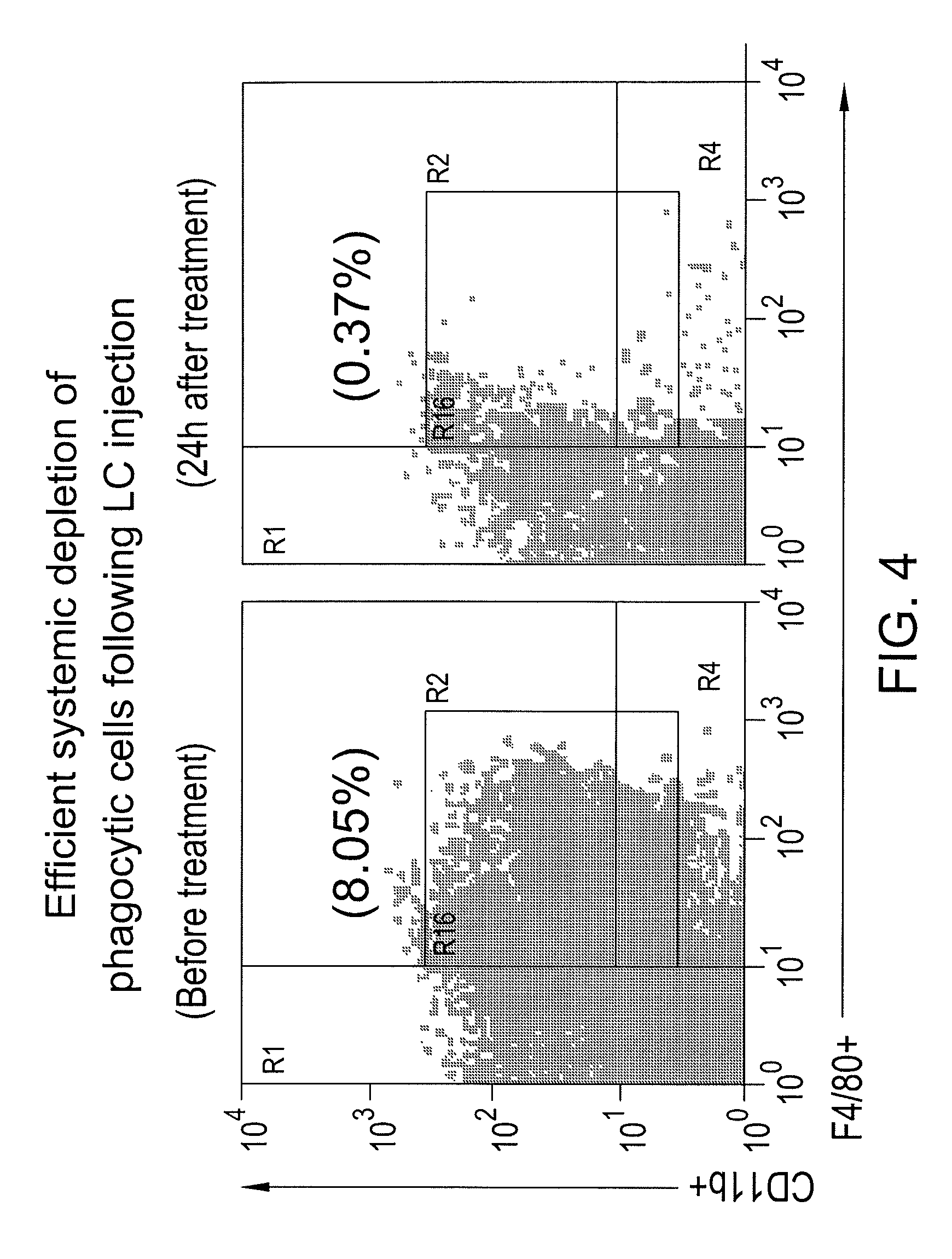
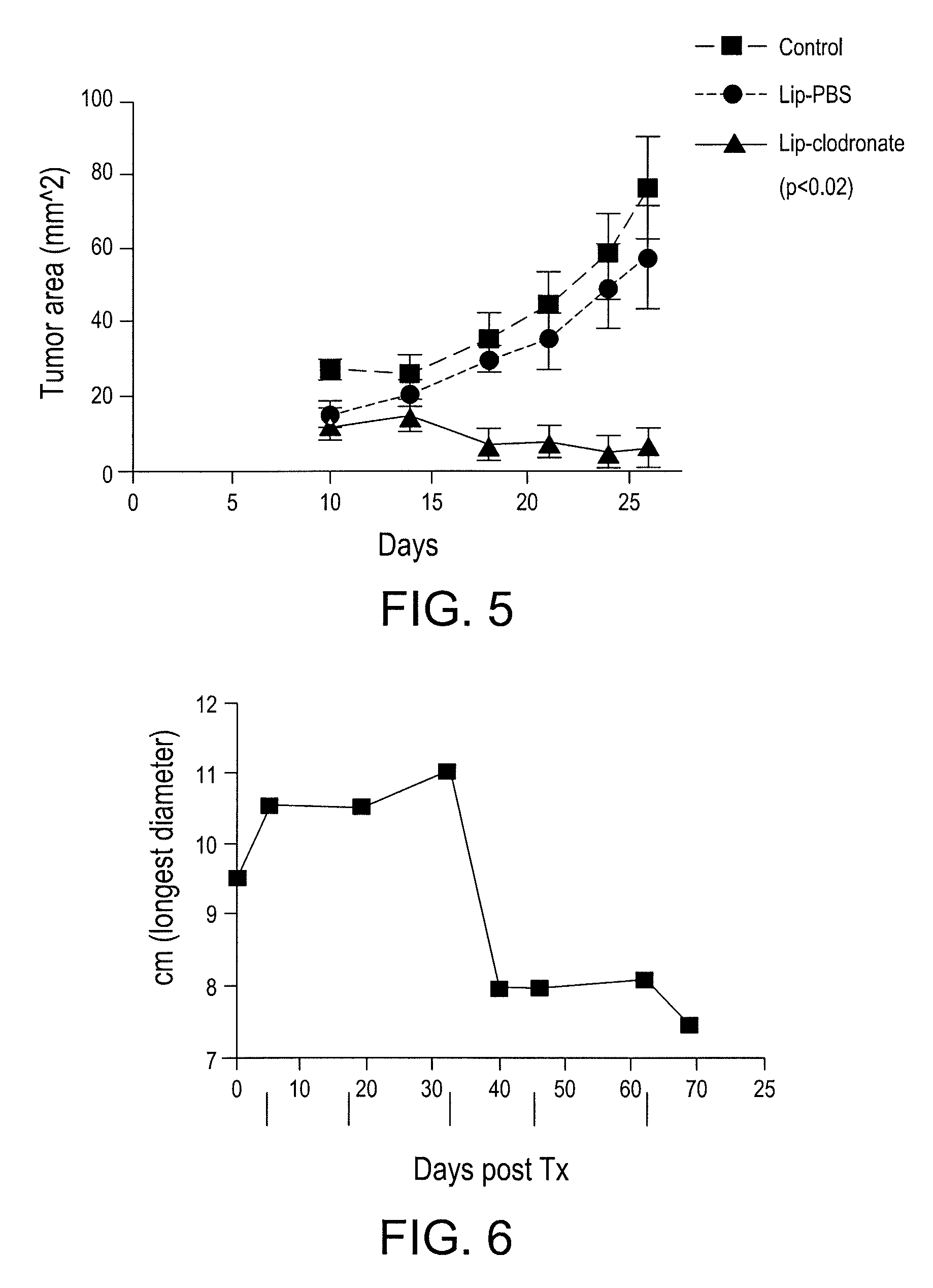
Myeloid derived suppressor cell (MDSC) inhibitory agents and vaccine and / or adjuvant enhancers are provided. Improved vaccine treatment regimens employing these agents are also provided. Cancer vaccines and methods for inhibiting tumor growth and cancer metastases are also presented. The myeloid derived suppressor cell (MDSC) inhibiting agents are described as bisphosphonates (such as liposomal clodronate) and CCR2 inhibitors and / or CCR2 antagonists. Methods for enhancing antibody titer levels in response to an antigen of interest are also provided.
Owner:COLORADO STATE UNIVERSITY
Cell death inducing agent for cells having braf gene mutation, growth suppressing agent for same cells and pharmaceutical composition for therapy of diseases caused by growth defect of same cells
ActiveUS20180135058A1Improve efficacyInduce cell deathCompound screeningOrganic active ingredientsGrowth retardantDisease
Owner:NITTO DENKO CORP
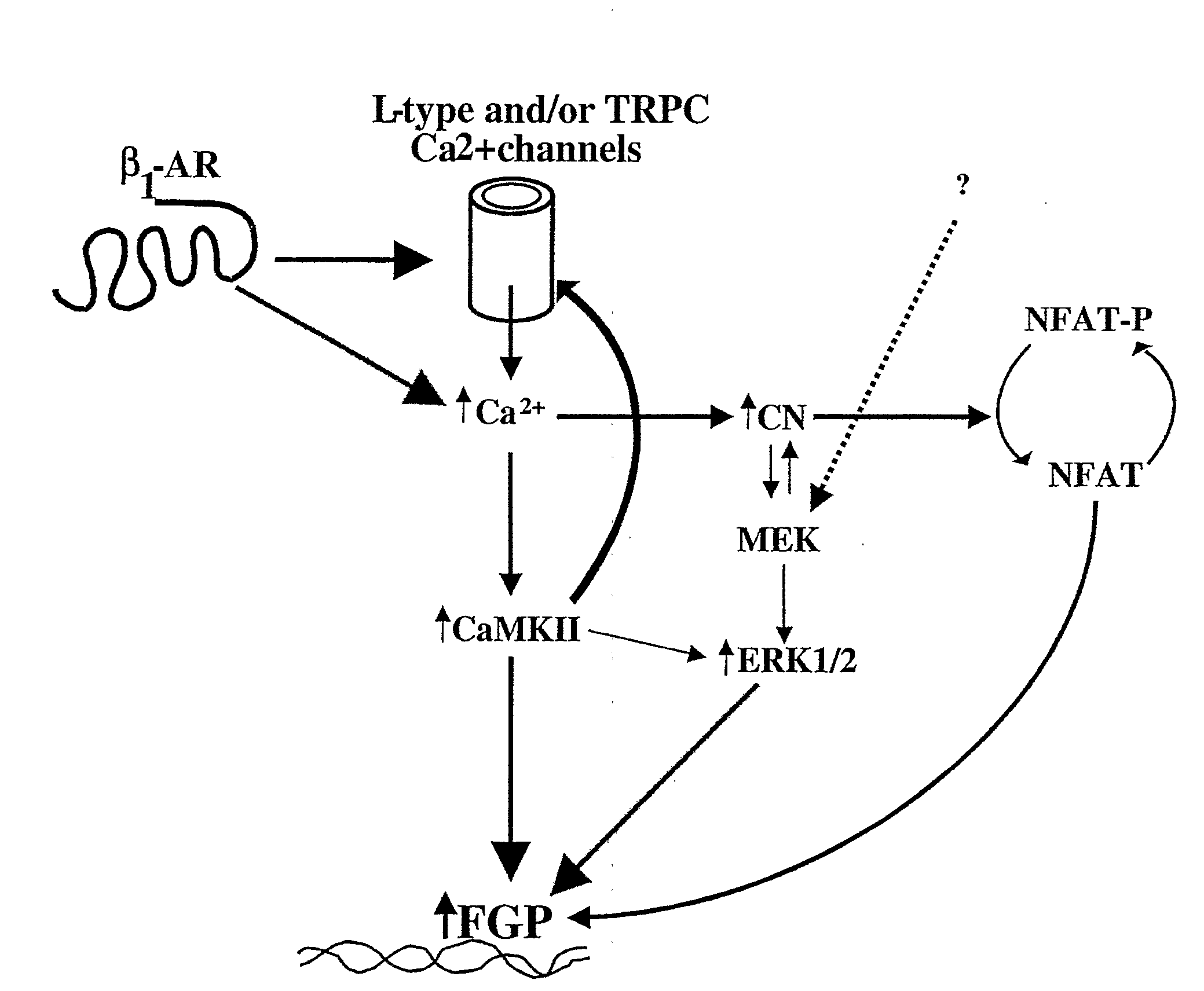
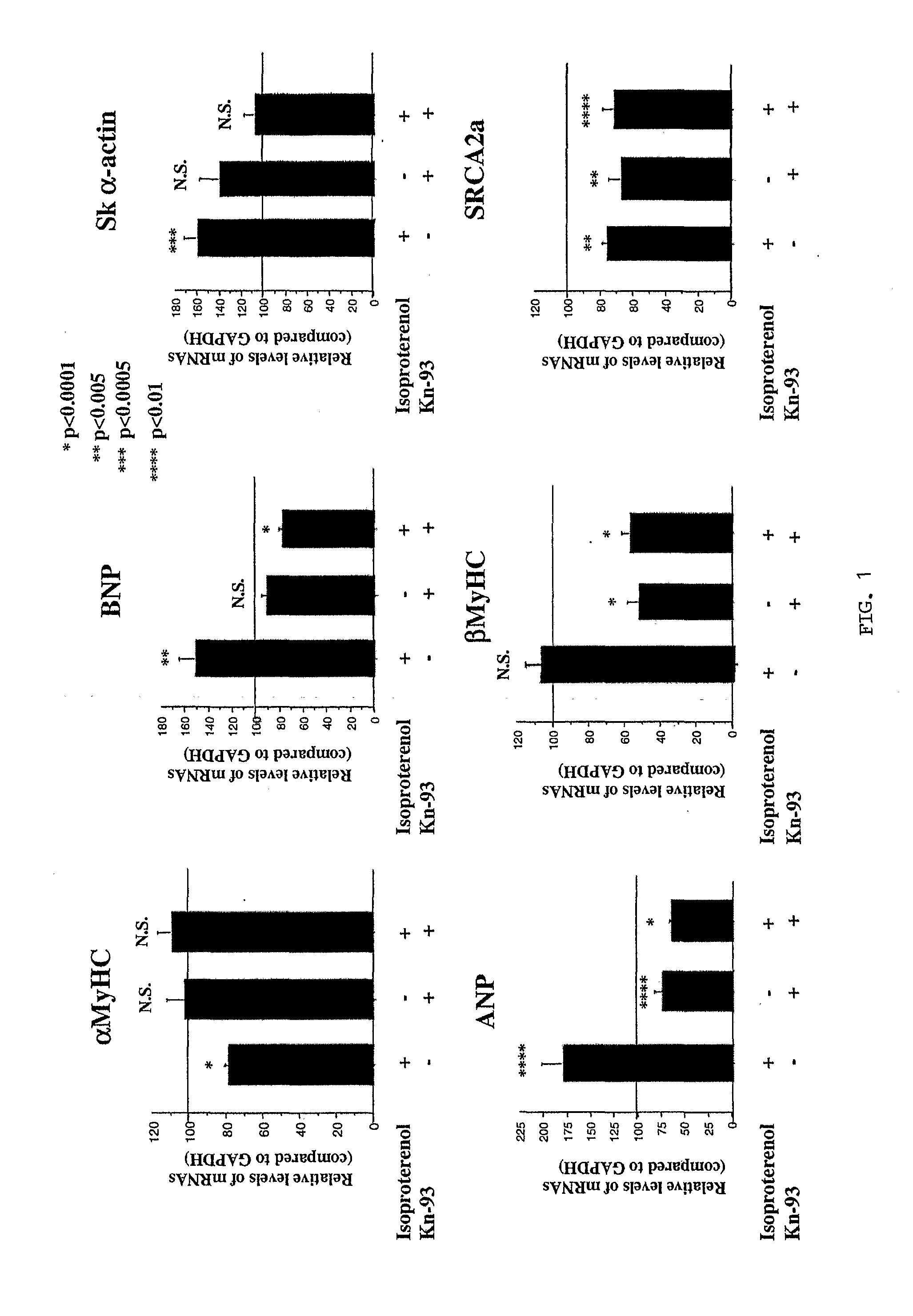
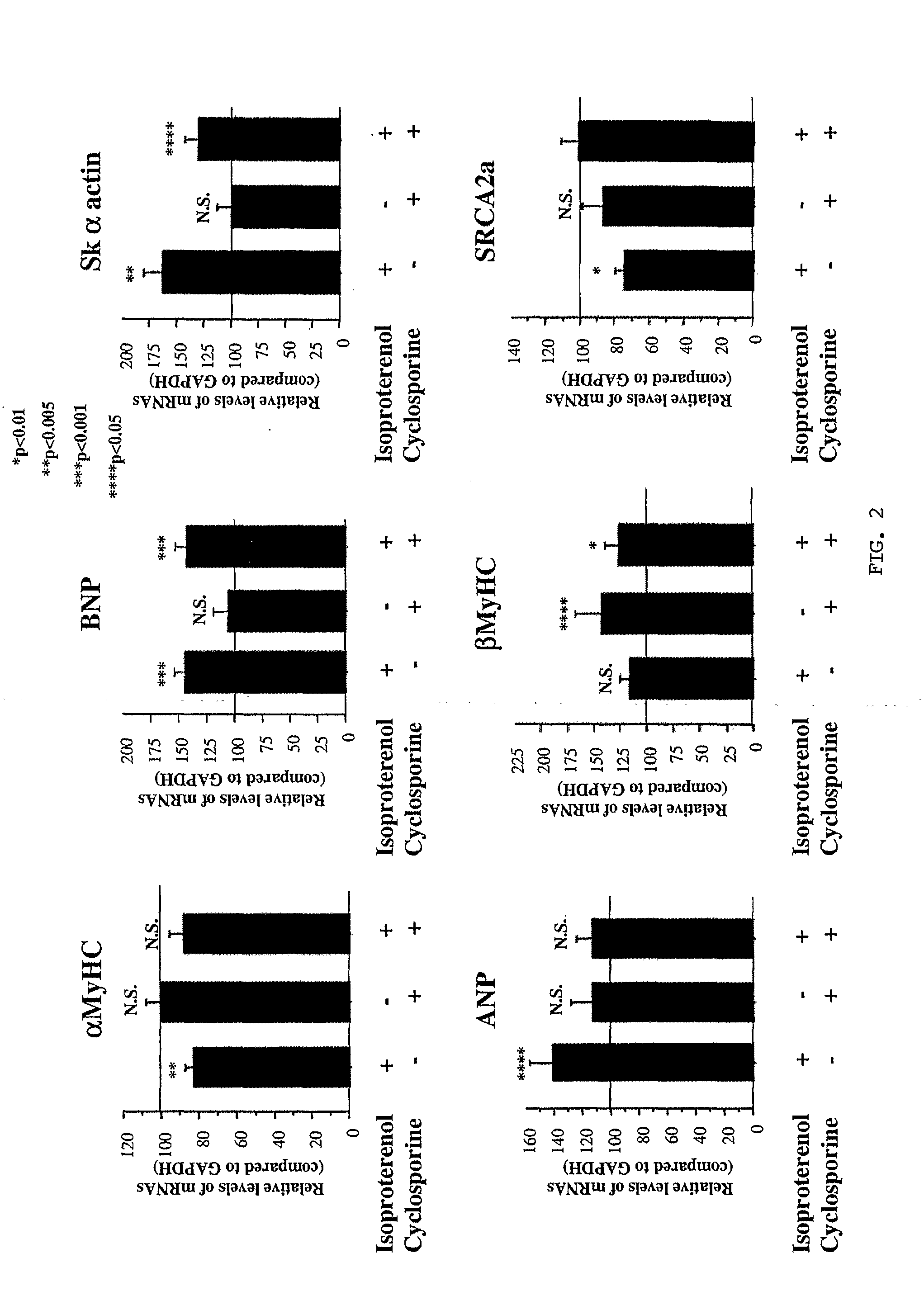
Inhibition of extracellular signal-regulated kinase 1/2 as a treatment for cardiac hypertrophy and heart failure
InactiveUS20090220507A1Improve athletic abilityIncrease injection volumeCompound screeningApoptosis detectionExtracellular signalManagement of heart failure
The present invention provides for methods of treating and preventing cardiac hypertrophy and heart failure. The present invention provides the link between ERK1 / 2 and calcineurin and CamKII. The present invention further demonstrates that inhibitors of ERK1 / 2 inhibit cardiac hypertrophy and heart disease by inhibiting, in part, the fetal cardiac gene expression that occurs when Ca2+-dependent signalling occurs in the heart.
Owner:UNIV OF COLORADO THE REGENTS OF
Methods and compositions relating to fortilin, an anti-apoptotic molecule, and modulators of fortilin
InactiveUS7691567B2Inhibit apoptosisReduces Fortilin activityNervous disorderPeptide/protein ingredientsAbnormal tissue growthNormal growth
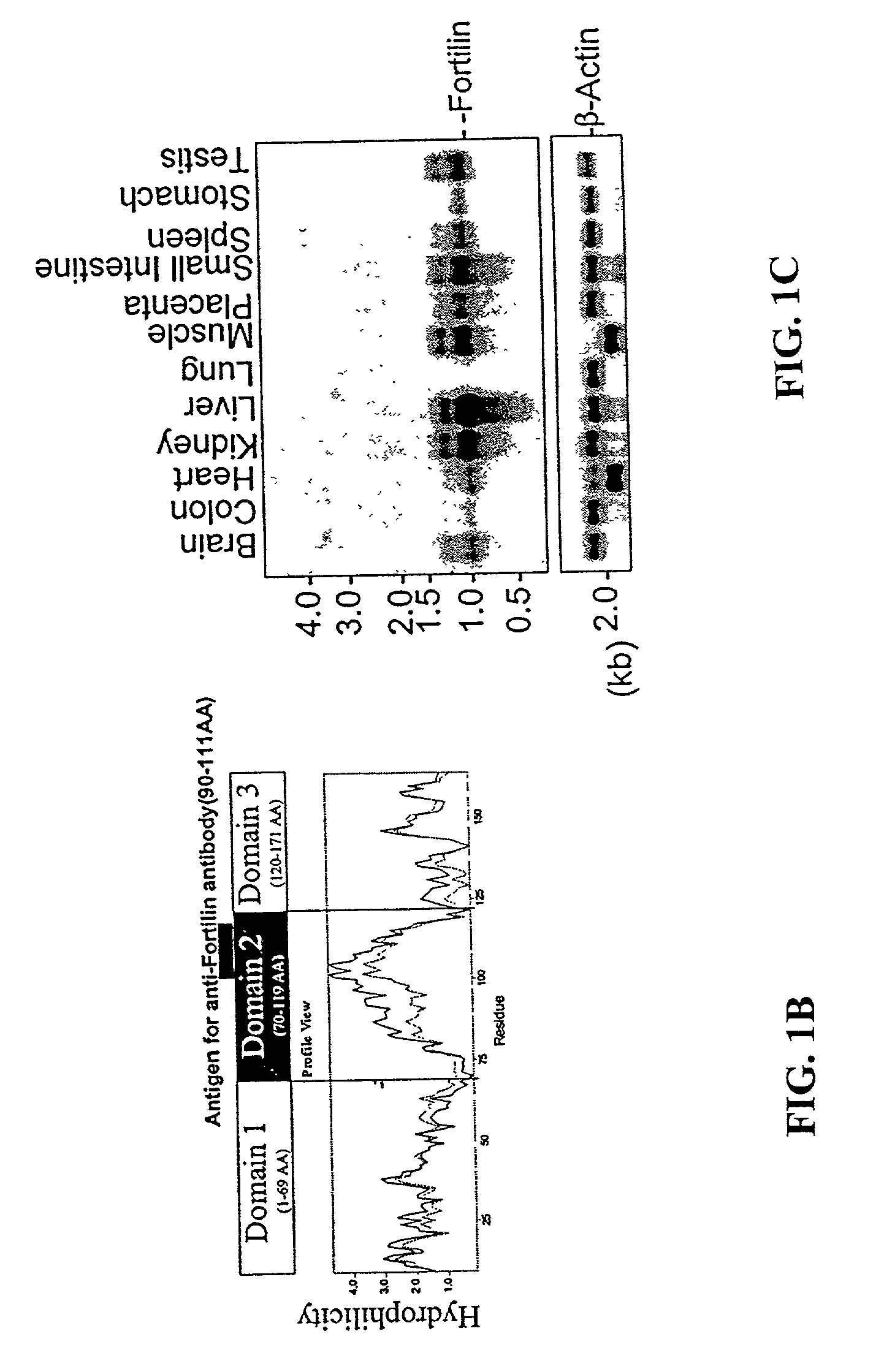
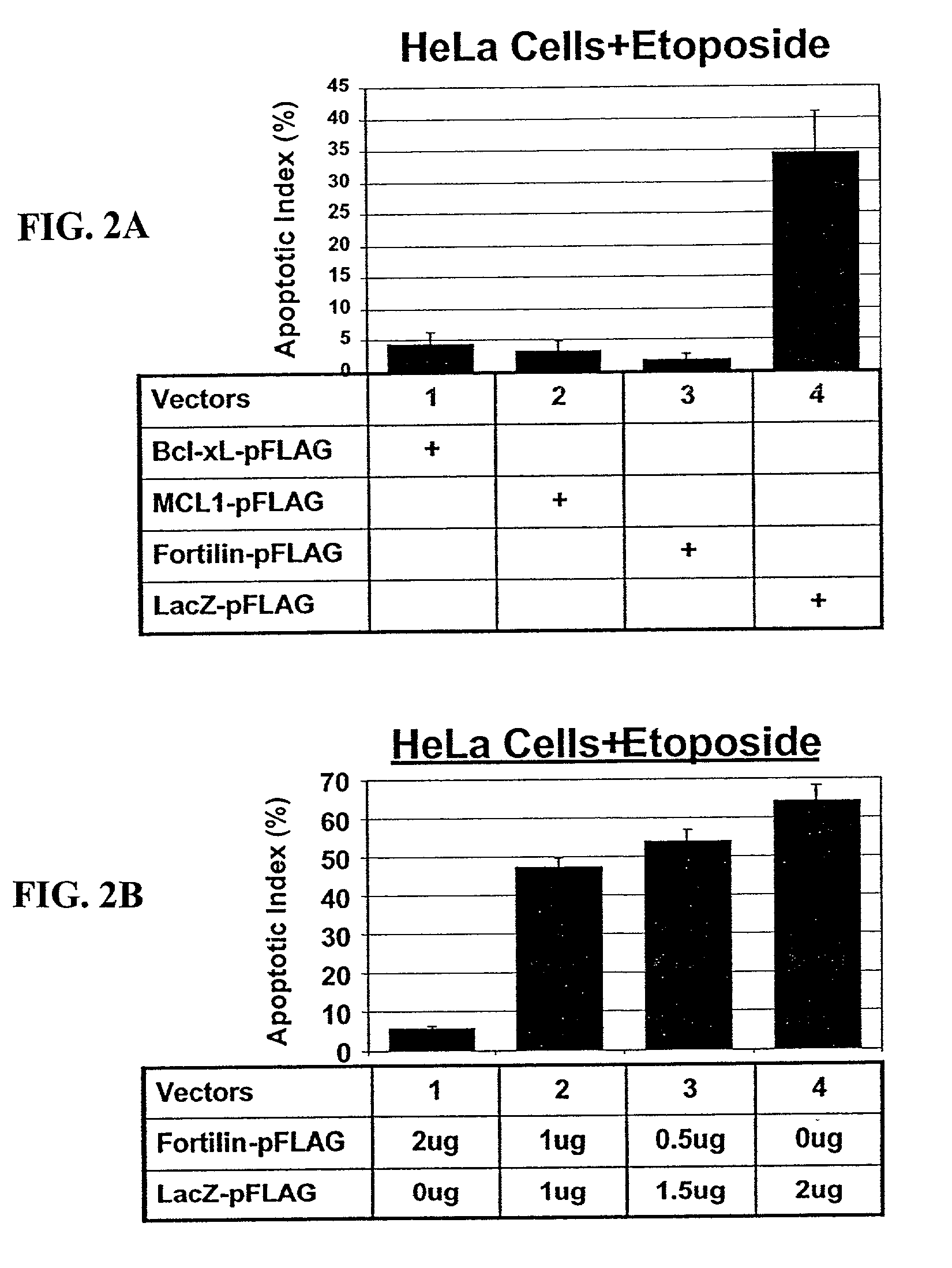
The polypeptide Fortilin (also known as Translationally Controlled Tumor Protein, TCTP) specifically interacts with p53, a tumor suppressor involved in the induction of apoptosis and the normal growth regulation of a cell. Fortilin also specifically binds MCL1 (Myeloid Cell Leukemia 1). Fortilin has the ability to prevent apoptosis, which may be unregulated in hyperproliferative cells. The present invention is directed at compositions and methods involving a Fortilin modulator, which can induce apoptosis, for the prevention, treatment, or diagnosis of hyperproliferative diseases and conditions, including cancer and atherosclerosis. It is directed also at compositions and methods involving Fortilin, which can inhibit apoptosis, for the treatment of diseases and condition characterized by apoptosis, including certain vascular conditions.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Tumor suppressor gene polypeptides and related nucleic acids, host cells, compositions, and methods of use in inhibition of cell growth, modulation of gene expression, and enhancement of immune-response inducing effect of a vaccine
InactiveUS20050164943A1Strong specificityGood effectPeptide/protein ingredientsImmunoglobulinsCancer cellNucleotide
A polypeptide consisting essentially of the amino acid sequence NCEHFVTYCRYG (SEQ ID NO: 1), ENCEHFVNELRYG (SEQ ID NO: 2), NCEHFVNELRYG (SEQ ID NO: 3), RNCEHFVAQLRYG (SEQ ID NO: 4), NCEHFVAQLRYG (SEQ ID NO: 5), or NCEHFVTYLRYG (SEQ ID: 6), or a variant of any of the foregoing, a composition comprising same, a nucleic acid consisting essentially of a nucleotide sequence encoding the amino acid sequence of any of SEQ ID NOS: 1-6 or a variant thereof, a composition comprising same, a host cell comprising an above-described nucleic acid molecule, a method of inhibiting cancerous cell growth comprising contacting a collection of cancerous cells with a cell growth-inhibiting effective amount of one or more of the foregoing, a method of modulating gene expression comprising contacting a collection of cells with a gene expression-modulating effective amount of at least one above-described nucleic acid or a composition comprising same, and a method of enhancing the immune response-inducing effect of a vaccine, which method comprises adding to the vaccine at least one above-described polypeptide, or a variant thereof.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
Parakeratosis inhibitor, pore-shrinking agent and skin preparation for external use
InactiveUS20050152930A1Increase acid concentrationBiocideCosmetic preparationsInhibitory cellN-Methyl-D-aspartic acid
It is intended to provide a substance having an effect of shrinking pores by analyzing the mechanism of making pores perceptible and compositions such as a skin preparation for external use which exerts the above effect to thereby make pores imperceptible. As means for solving these problems, there are provided a parakeratosis inhibitor and a pore-shrinking agent comprising an antagonist to an excitatory cell receptor, for example, a glutamate receptor such as N-methyl-D-aspartic acid receptor or an ATP receptor such as P2X receptor, or an agonist to an inhibitory cell receptor such as a γ-aminobutyrate receptor such as bicuculline-sensitive receptor having the Cl— channel therein or glycine receptor, as well as a skin preparation for external use aiming at inhibiting parakeratosis and a skin preparation for external use aiming at shrinking pores each containing such an antagonist to an excitatory cell receptor or an agonist to an inhibitory cell receptor as described above. Owing to the effects of inhibiting parakeratosis and shrinking pores, the skin can be maintained in a healthy state without perceptible pores.
Owner:SHISEIDO CO LTD
Methods for attenuating release of inflammatory mediators and peptides useful therein
The present invention includes methods of inhibiting or suppressing cellular secretory processes. More specifically the present invention relates to inhibiting or reducing the release of inflammatory mediators from inflammatory cells by inhibiting the mechanism associated with the release of inflammatory mediators from granules in inflammatory cells. In this regard, the present invention discloses an intracellular signaling mechanism that illustrates several novel intracellular targets for pharmacological intervention in disorders involving secretion of inflammatory mediators from vesicles in inflammatory cells. Peptide fragments and variants thereof of MANS peptide as disclosed in the present invention are useful in such methods.
Owner:BIOMARK PHARMACEUTICALS LTD
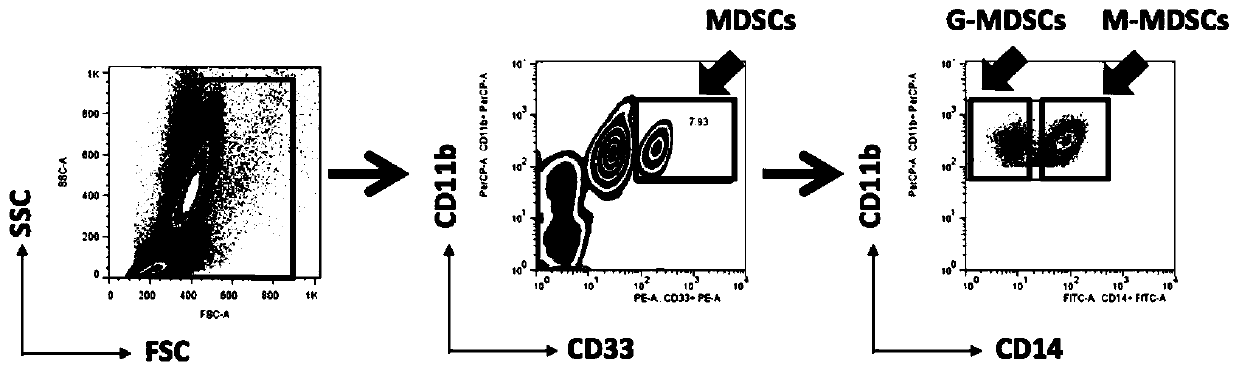
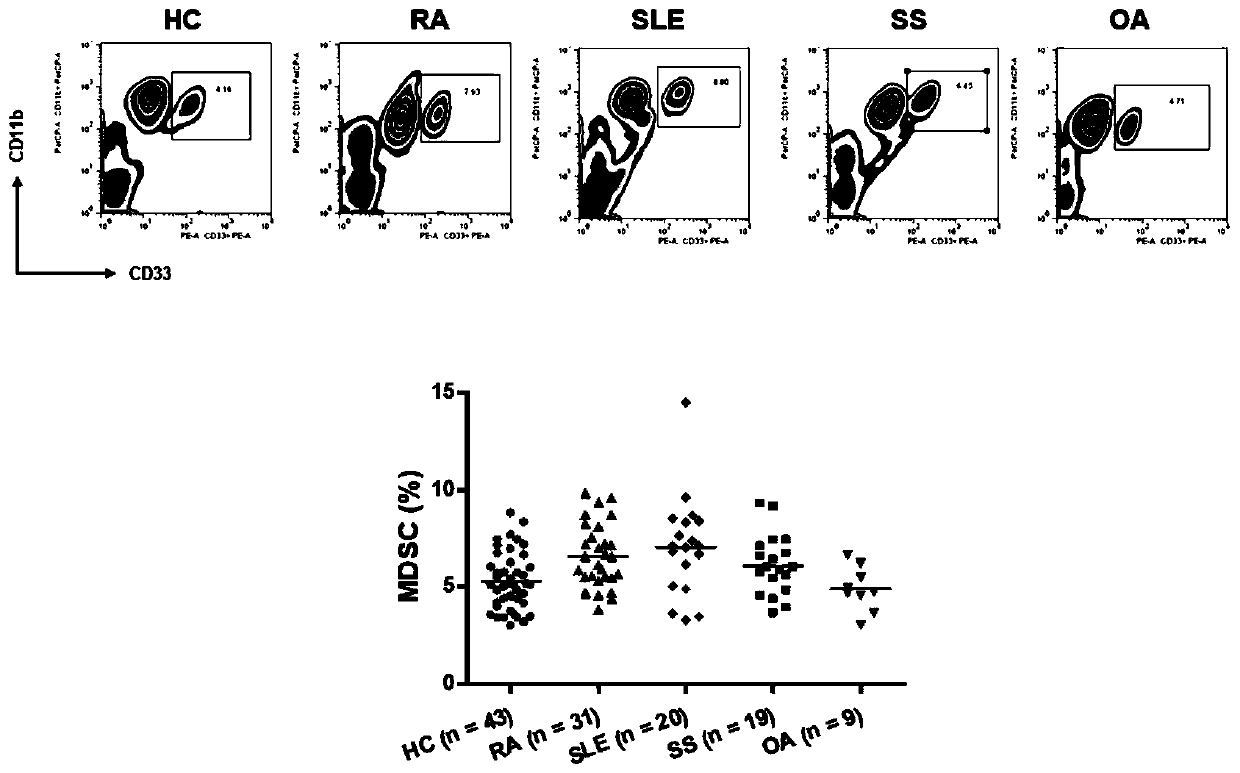
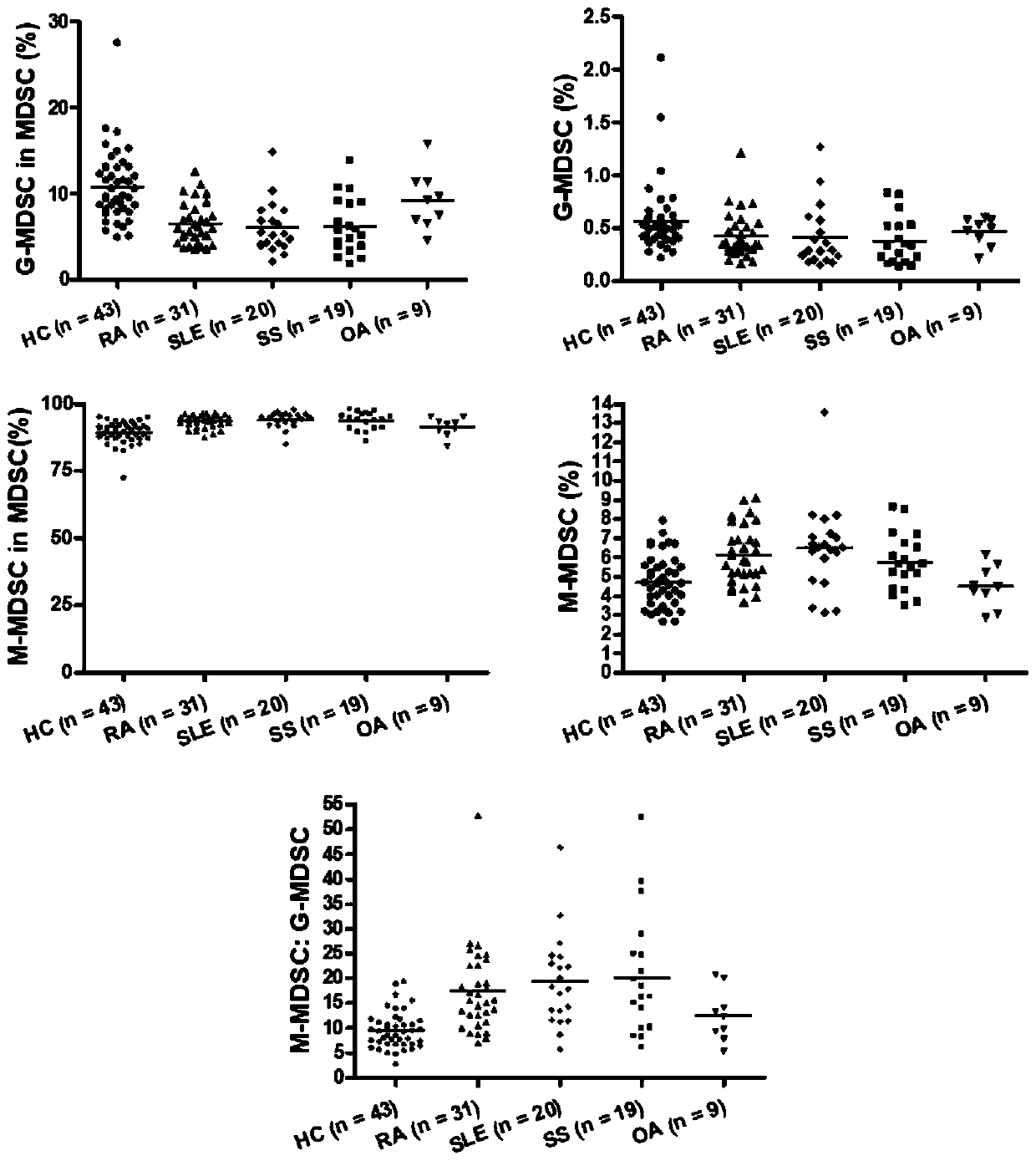
Application of inhibitory cells derived from medullary system in preparation of diagnostic reagents for autoimmune diseases
PendingCN109929902AEasy to detectImprove accuracyMicrobiological testing/measurementBiological testingAutoimmune conditionNormal people
The invention discloses application of inhibitory cells derived from a medullary system in preparation of diagnostic reagents for autoimmune diseases. It is found that MDSCs and its subgroup G-MDSCs,M-MDSCs are obviously different in normal people and patients with autoimmune diseases, and only peripheral blood and / or joint fluid sampling is needed, the ratio can be judged by flow cytometry, a specific value is calculated, and the detection is very simple and convenient; in addition, by setting a cut-off value (mean value of normal human + 3SD), higher accuracy can be further ensured, the autoimmune disease can be determined without comprehensive judgment combined with other markers, good indication can be provided for diagnosis and drug treatment effects of the autoimmune disease, and clinical application is facilitated.
Owner:PEOPLES HOSPITAL PEKING UNIV
Inhibitors of cell adhesion
ActiveUS20170335404A1Limit and block cancer metastasisInhibiting adhesion of cellImmunoglobulin superfamilyMicrobiological testing/measurementAdhesion processCell adhesion
Owner:RGT UNIV OF MINNESOTA
Application of berberine to preparing drug for preventing and treating familial adenomatous polyp
InactiveCN102058590AGood control effectNo obvious side effectsOrganic active ingredientsDigestive systemApoptosisBiological activation
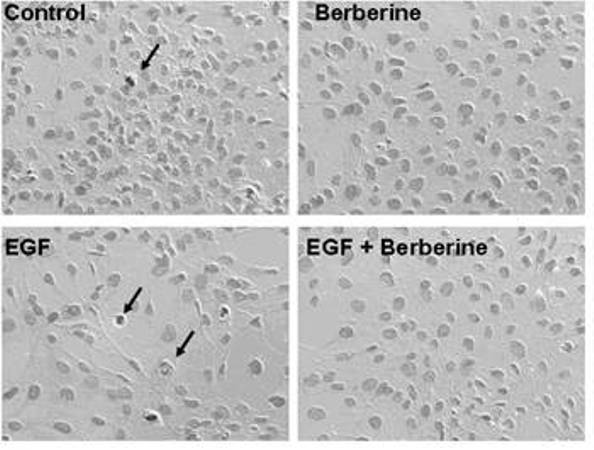
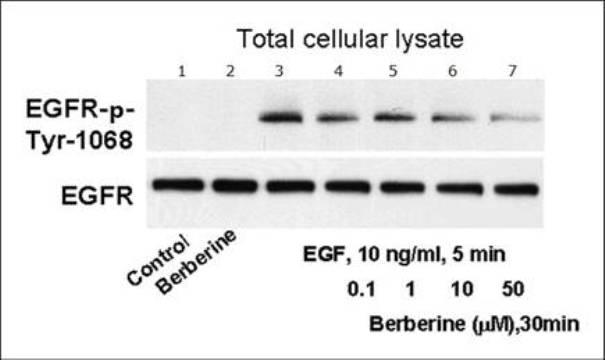
The invention relates to novel application of berberine, in particular to application of the berberine to preparing a drug for preventing and treating familial adenomatous polyp (FAP). The FAP is an autosomal dominant hereditary disease which is characterized by multiple adenomatous polyps in the digestive tract, mainly comprises CFAP (Classical Familial Adenomatous Polyp) and AFAP (Attenuated Familial Adenomatous Polyp) and is not rare in clinic; and early intervention is significant. In the invention, the applied berberine can bring inhibitory action to the cell growth of colon cancer cells and FAP cell line (IMCE), wherein the mechanism includes inhibiting cell proliferation, promoting apoptosis, down regulating expression of COX-2 and AP-1, inhibiting synthesis of PGE2 (Prostaglandin E2) and activation of EGFR (Epidermal Growth Factor Receptor) and Akt and brings out well effect when applied to FAP patients in a long term. The invention provides a novel safe, effective, and economic method for preventing and treating the FAP.
Owner:GENERAL HOSPITAL OF TIANJIN MEDICAL UNIV
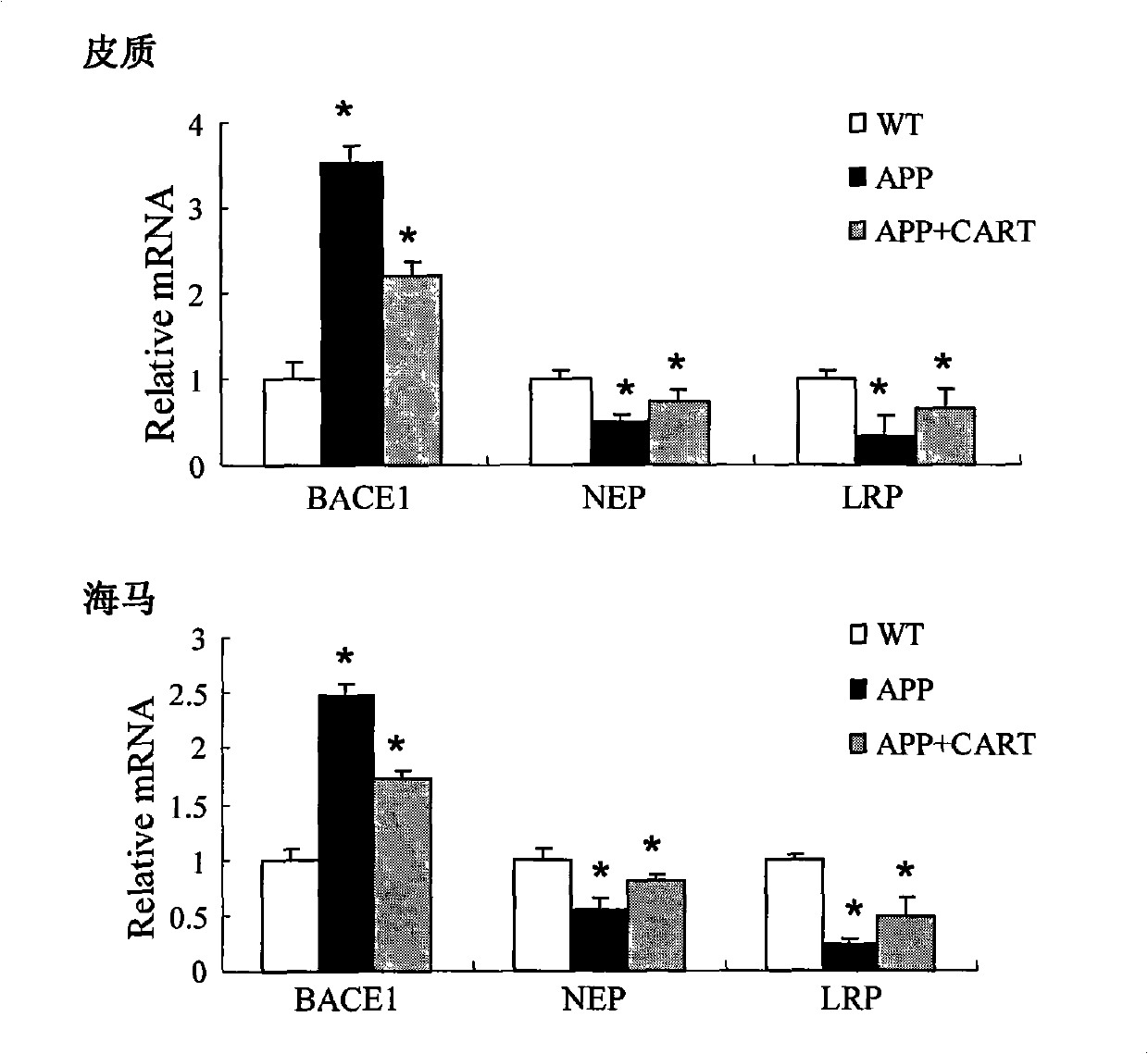
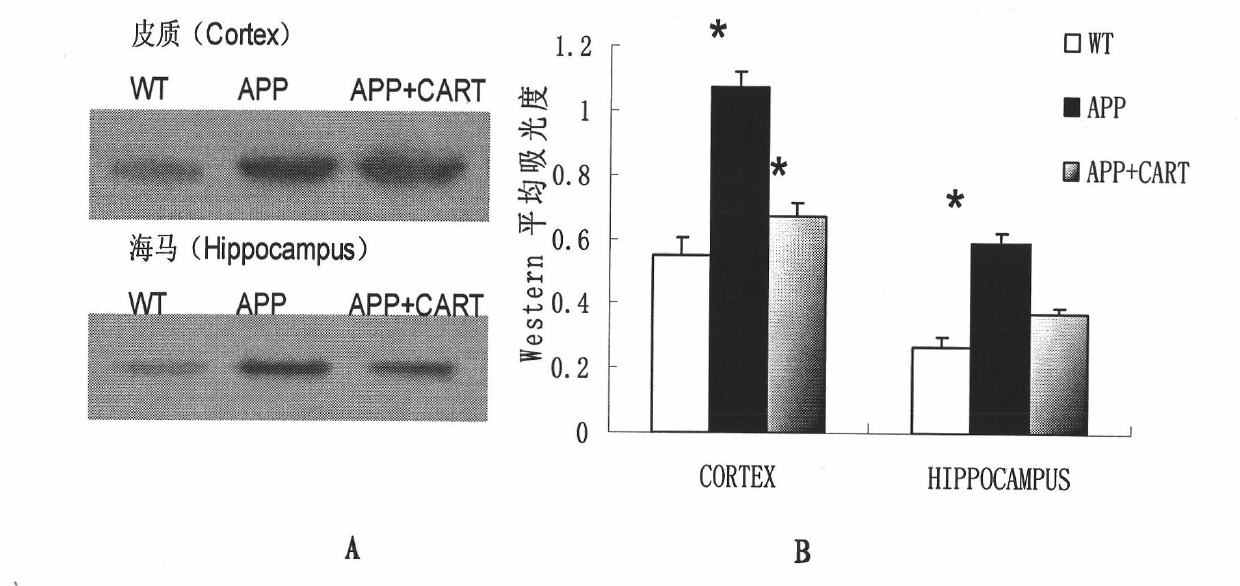
Application of CART (Cocaine Amphetamine Regulated Transcript) in preparing medicaments for treating alzheimers diseases
InactiveCN101874895AReduce formationHigh expressionNervous disorderPeptide/protein ingredientsMembrane potentialSuccinate dehydrogenase
The invention relates to application of CART (Cocaine Amphetamine Regulated Transcript) in preparing medicaments for treating alzheimers diseases. In the invention, CART is prepared into injection preparations for treating alzheimers diseases, and the defects of the traditional medicaments for enhancing cholinergic action and N-methyl-D-aspartic acid (NMDA) receptor antagonists are overcome. The novel medicament has multiple target point actions on the generation and the degradation of A beta as well as the influence of the neurotoxicity and the molecular mechanism of the A beta. The invention has effects on effectively treating dementia with estrogen and up-regulating intracephalic CART; more CARTs are distributed in intracephalic hippocampus; CART exists in intracephalic acetylcholine neurons and can promote the release of acetylcholine; and mitochondrial dysfunction induced by A beta neurotoxicity is the main pathway for the A beta to cause neuronal apoptosis. The CART has a directaction on mitochondrial succinodehydrogenase subunit B and can maintain the respiration of mitochondria and the generation of energy, alleviate oxidative stress, stabilize mitochondrial membrane potential and prevent the mitochondria from being damaged, thereby inhibiting apoptosis.
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
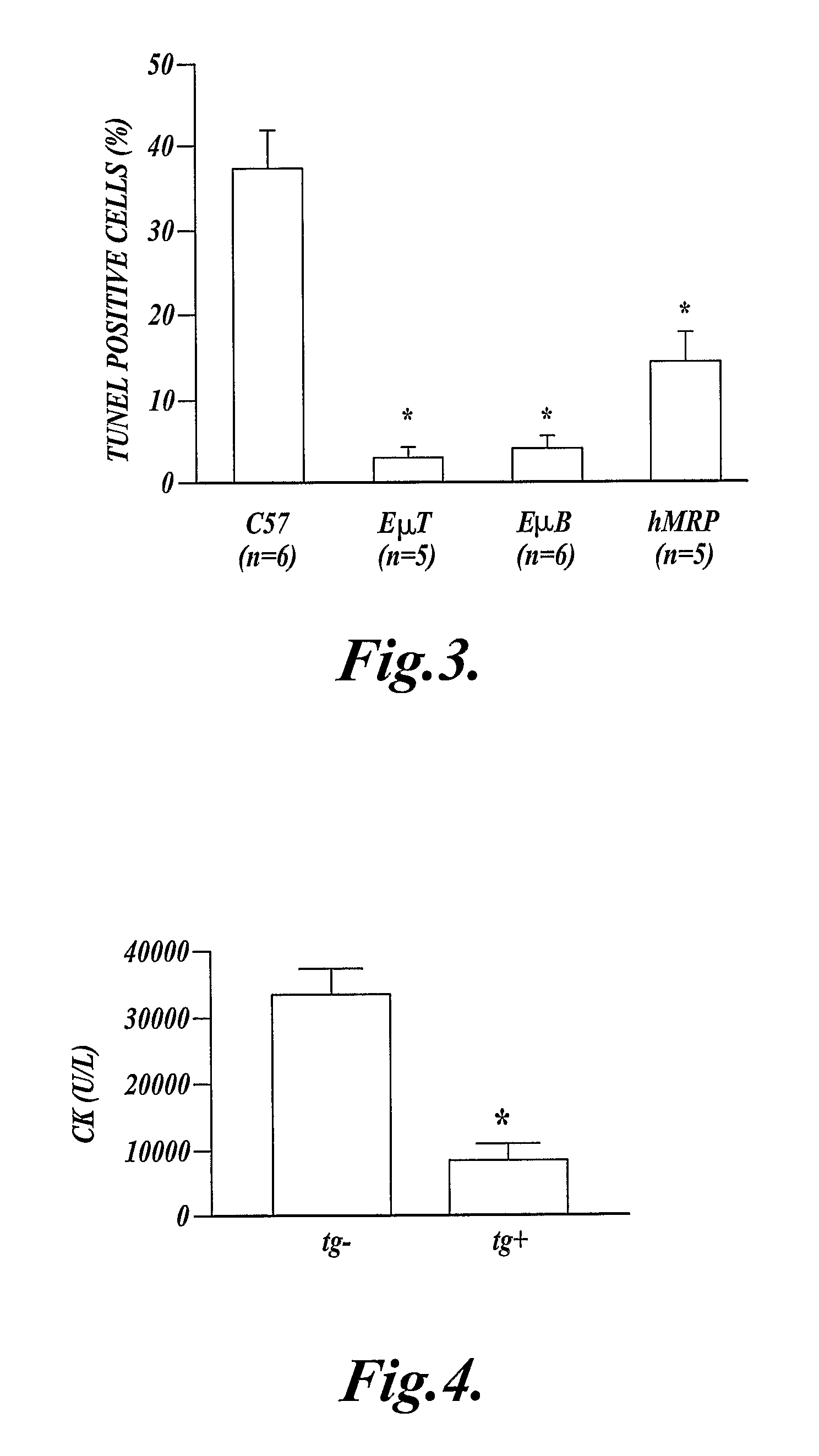
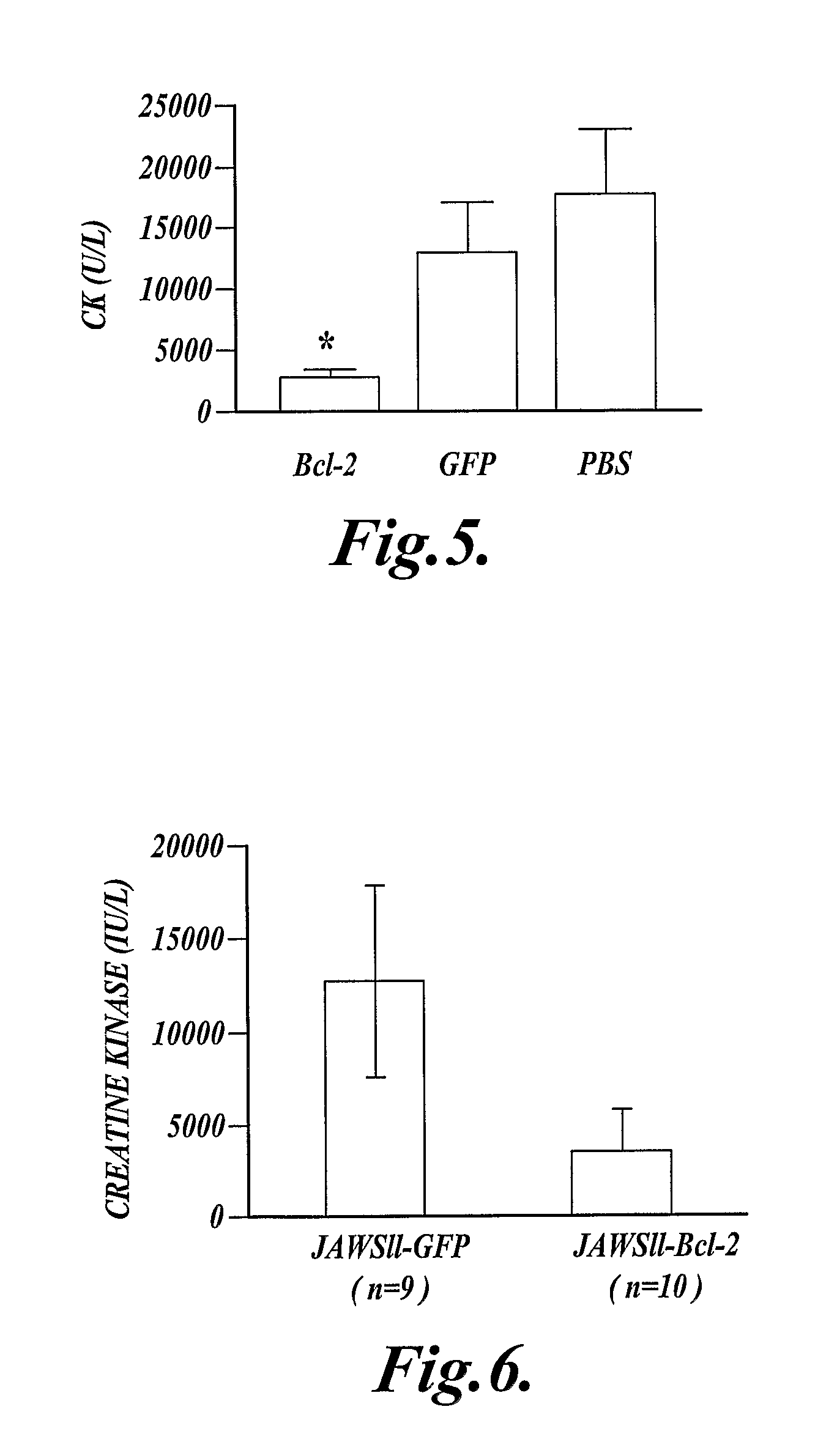
Methods of Inhibiting Cell Death or Inflammation in a Mammal
InactiveUS20110082072A1Avoid cell deathInhibit inflammationAntibacterial agentsPeptide/protein ingredientsBiologyInflammation
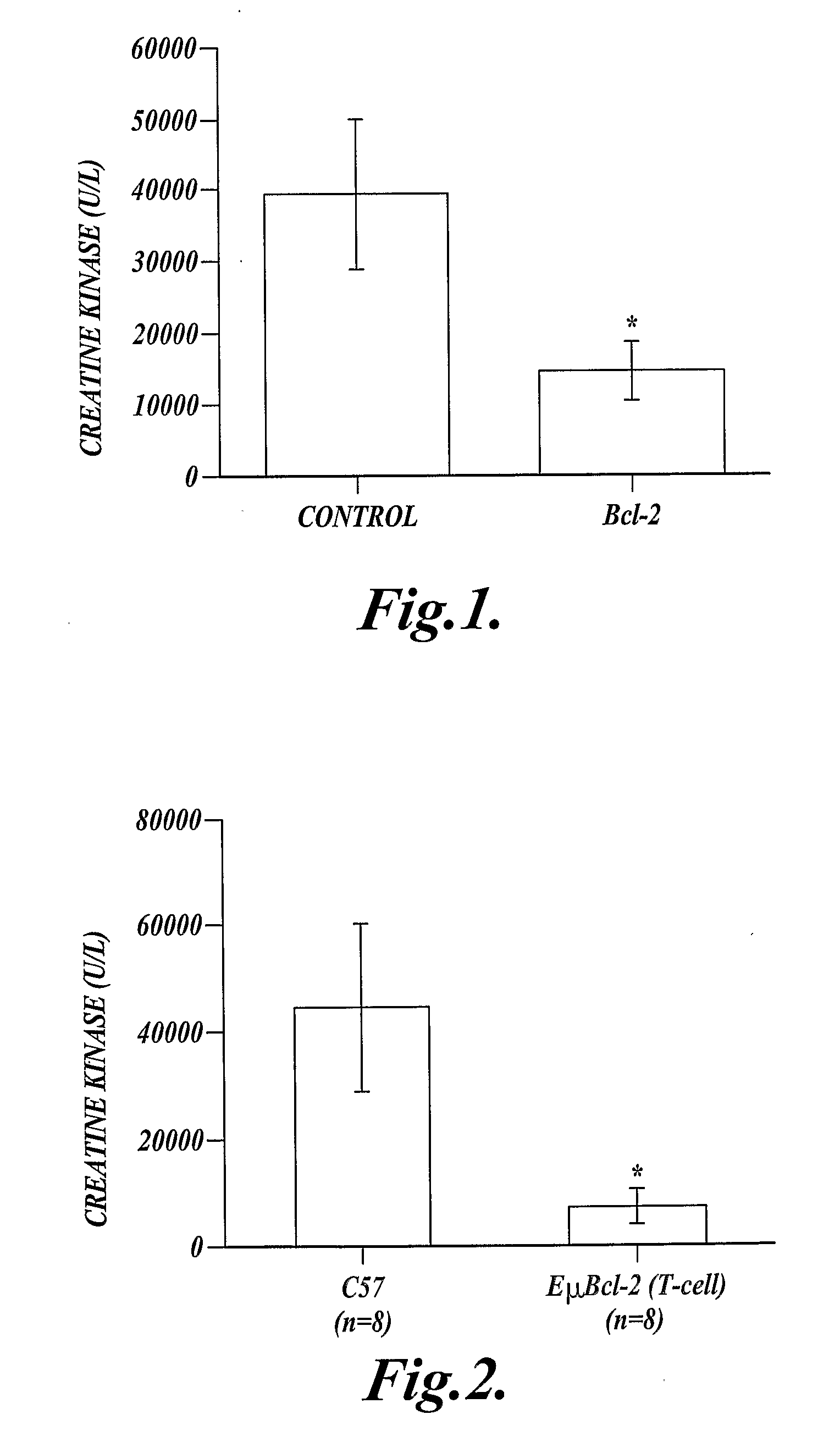
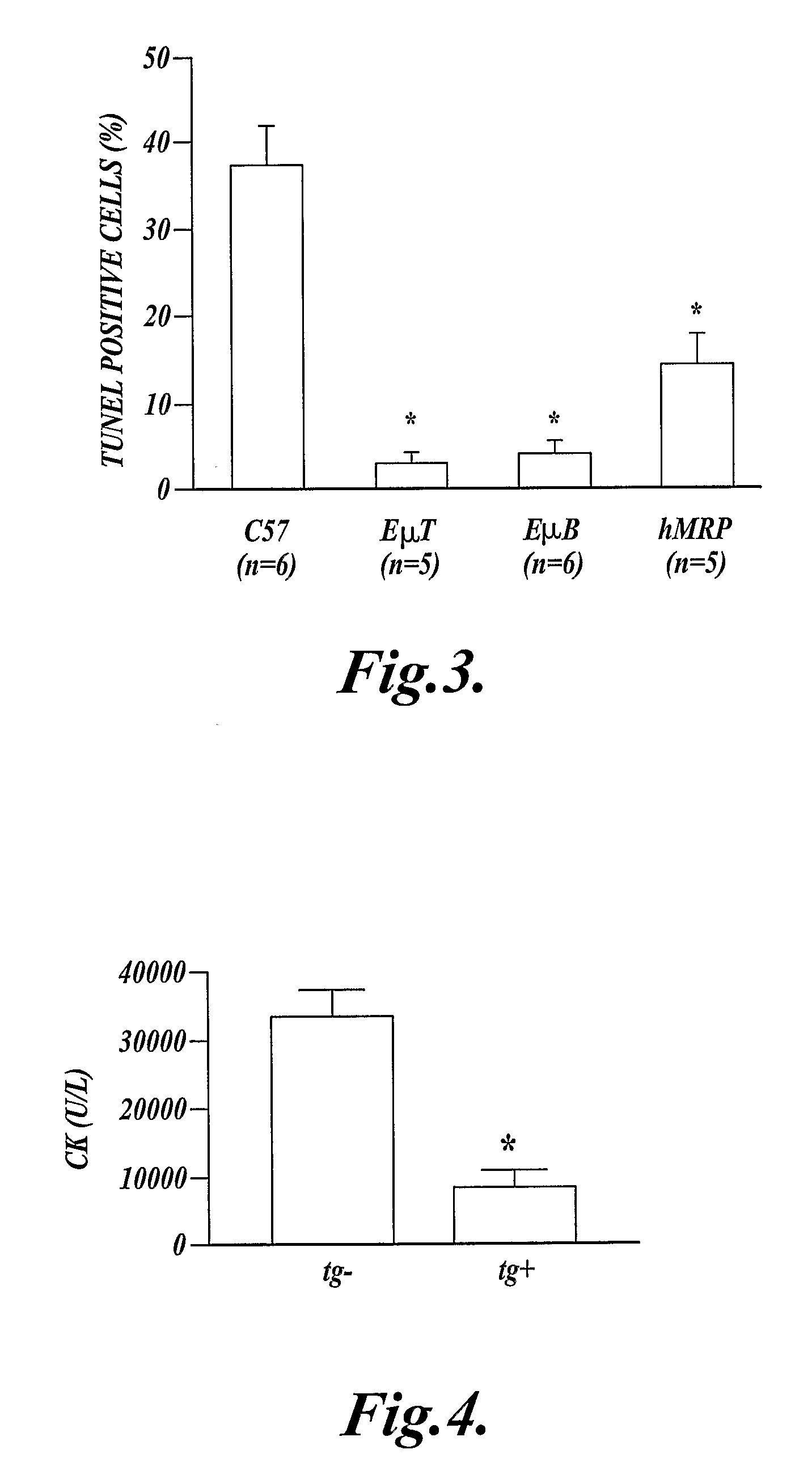
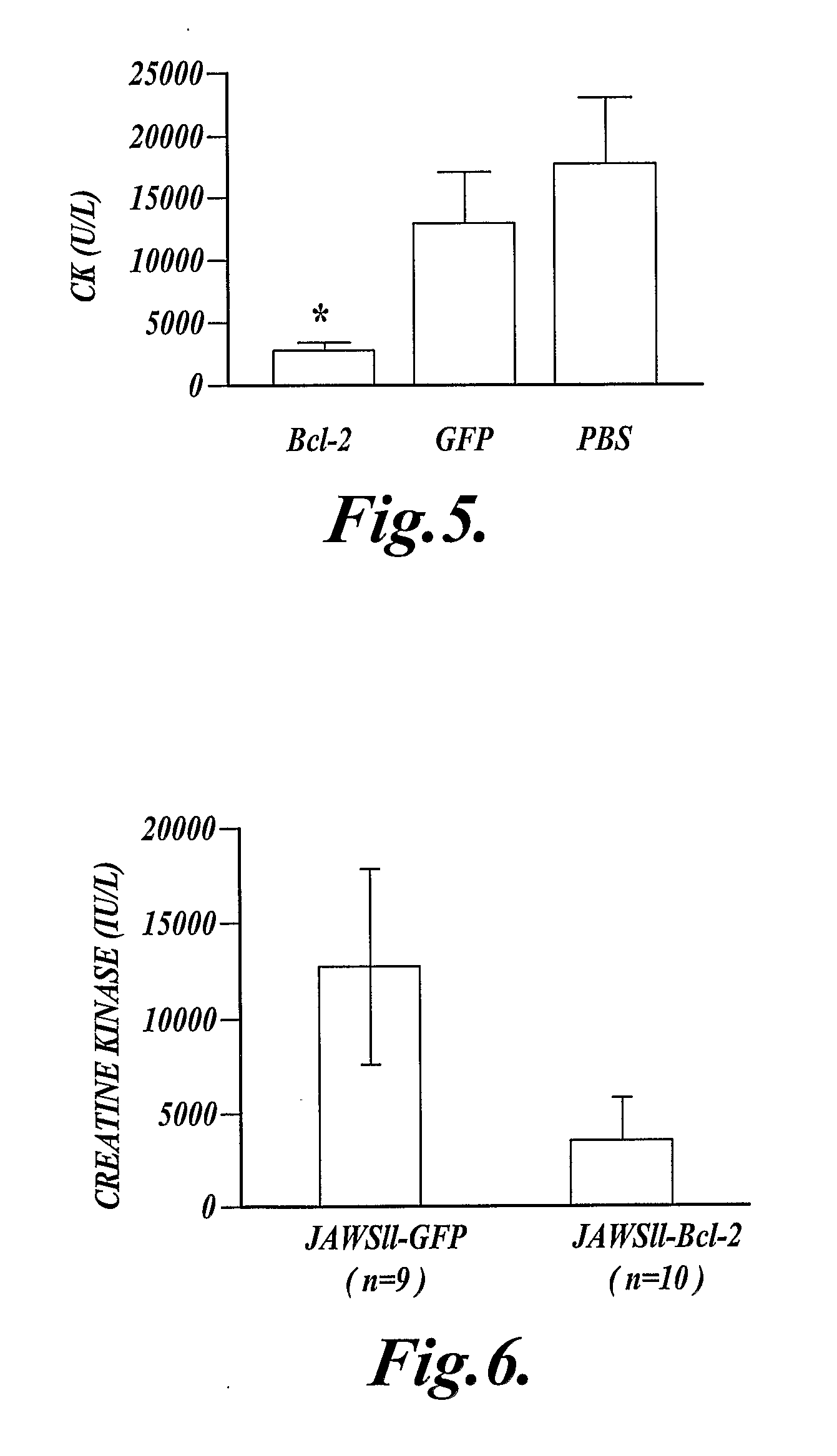
In one aspect the present invention provides methods for inhibiting cell death or inflammation in a mammal, wherein the methods each include the step of administering to a mammal a Bcl protein in an amount sufficient to inhibit cell death or inflammation in the mammal. The invention also provides methods for identifying a Bcl protein that inhibits cell death or inflammation when administered to a mammal.
Owner:UNIV OF WASHINGTON
Complex immuno-gene medical composition for inhibiting tumor cells
ActiveUS20050203037A1Growth inhibitionRestoring cytotocicitySugar derivativesPeptide/protein ingredientsNatural Killer Cell Inhibitory ReceptorsTh2 cytokines
Disclosed is a complex immuno-gene medical composition activating NK cells to enhance host immune system. The composition is usage of a combination of a plurality of cytokines, combined usage of the kinds of Th1 and Th2 cytokines. Th2 cytokine antagonizes TGF-β inhibiting NK cells to disable the inhibition of immune system, and Th1 cytokine activates NK cells in host to enhance the ability fighting against tumor cells. By means of the complex immuno-gene medical composition, removal of tumor cells is expectable.
Owner:NAT TAIWAN UNIV
Method of inhibiting inflammation in a mammal by administering Bcl protein
In one aspect the present invention provides methods for inhibiting cell death or inflammation in a mammal, wherein the methods each include the step of administering to a mammal a Bcl protein in an amount sufficient to inhibit cell death or inflammation in the mammal. The invention also provides methods for identifying a Bcl protein that inhibits cell death or inflammation when administered to a mammal.
Owner:UNIV OF WASHINGTON
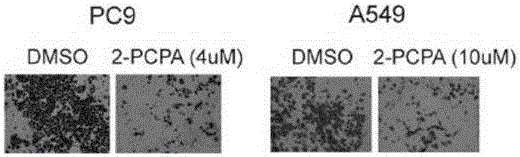
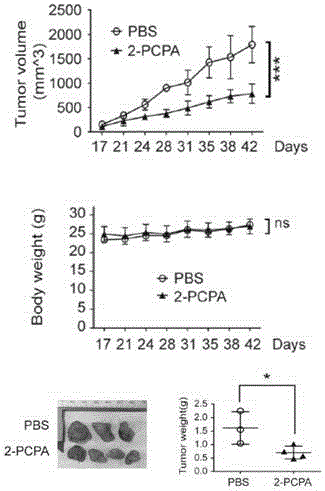
Application of KDM1A micro-molecular inhibitor in inhibition of growth and transfer of tumor cells
InactiveCN106727461AGood treatment effectProlong lifeOrganic active ingredientsAntineoplastic agentsTreatment of lung cancerTherapeutic effect
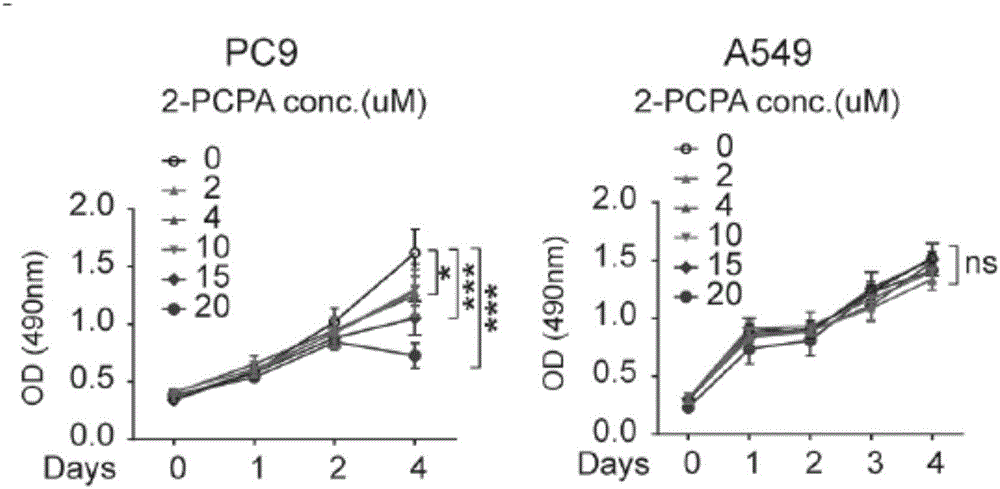
The invention relates to an application of a KDM1A micro-molecular inhibitor in the inhibition of the growth and the transfer of tumor cells. The KDM1A micro-molecular inhibitor can inhibit the activity of histone demethylase KDM1A in cells and inhibit demethylation of histone H3K4me2 in order to cause up-regulation of the TIMP3 expression and promote down-regulation of extracellular MMP2 expression, inhibits the activity of JNK kinase in the cells, and effectively inhibits the invasion and the transfer of tumor cells; and the KDM1A micro-molecular inhibitor is 2-PCPA. The lung cancer cell proliferation, migration and invasion inhibition molecular mechanism of the KDM1A micro-molecular inhibitor 2-PCPA is explicated in the invention, the lung cancer treatment effect of the KDM1A micro-molecular inhibitor is researched on the basis of the molecular mechanism, the gap in the field of lung cancer treatment using an epigenetic medicine is filled, and the 2-PCPA can be used for preparing micro-molecular medicines for inhibiting the proliferation and the transfer of lung cancer cells, so the lung cancer treatment effect is improved, and the life of lung cancer patients is prolonged.
Owner:SHANGHAI PULMONARY HOSPITAL
Parakeratosis inhibitor, pore-shrinking agent and skin preparation for external use
It is intended to provide a substance having an effect of shrinking pores by analyzing the mechanism of making pores perceptible and compositions such as a skin preparation for external use which exerts the above effect to thereby make pores imperceptible. As means for solving these problems, there are provided a parakeratosis inhibitor and a pore-shrinking agent comprising an antagonist to an excitatory cell receptor, for example, a glutamate receptor such as N-methyl-D-aspartic acid receptor or an ATP receptor such as P2X receptor, or an agonist to an inhibitory cell receptor such as a γ-aminobutyrate receptor such as bicuculline-sensitive receptor having the Cl-channel therein or glycine receptor, as well as a skin preparation for external use aiming at inhibiting parakeratosis and a skin preparation for external use aiming at shrinking pores each containing such an antagonist to an excitatory cell receptor or an agonist to an inhibitory cell receptor as described above. Owing to the effects of inhibiting parakeratosis and shrinking pores, the skin can be maintained in a healthy state without perceptible pores.
Owner:SHISEIDO CO LTD
Application of hypoxia-inducible factor prolyl hydroxylase activity inhibitor in preparation of drug for preventing and treating acute kidney injury
ActiveCN108434139AInhibit apoptosisImprove pathological damageOrganic active ingredientsUrinary disorderHIF FamilyInflammatory factors
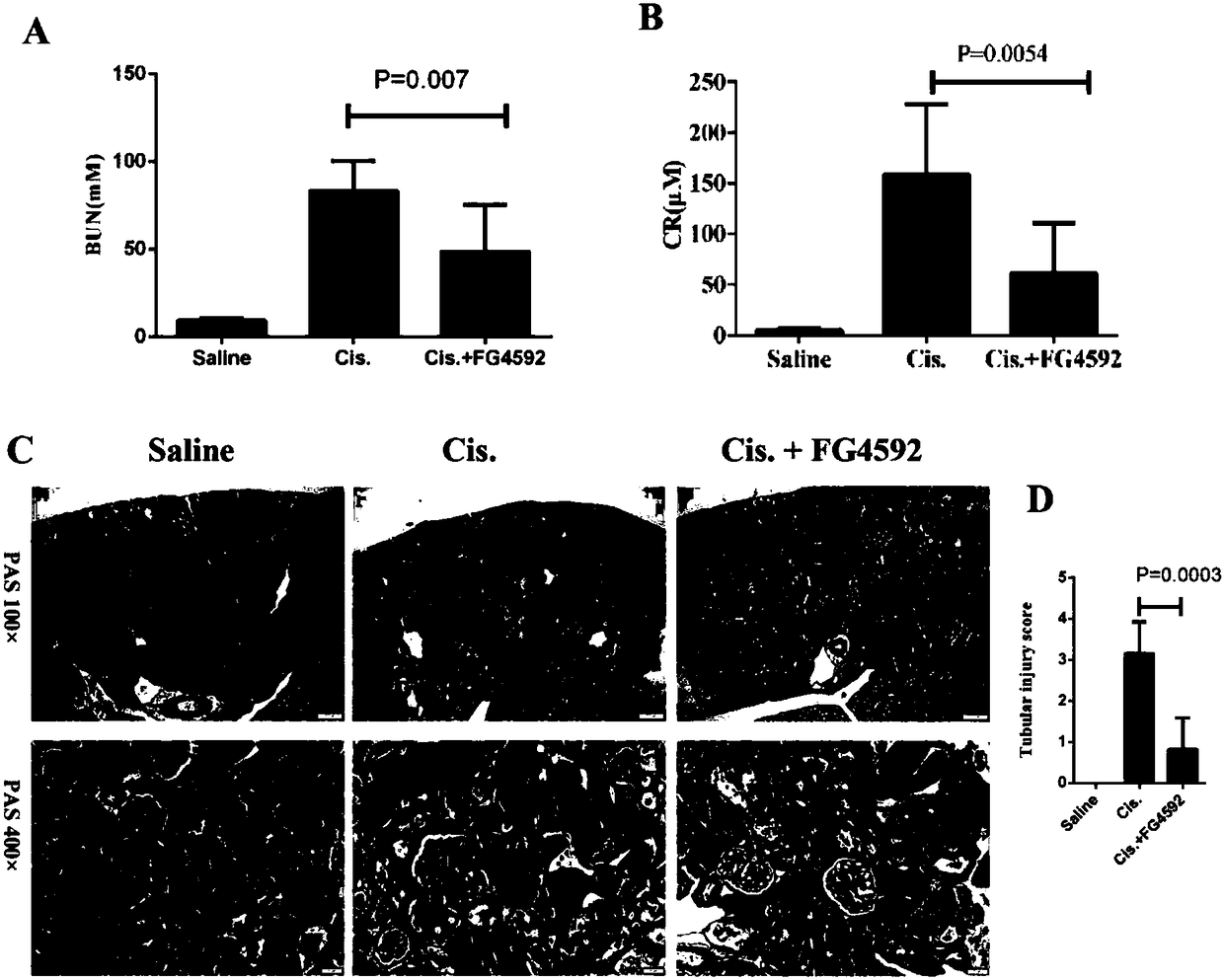
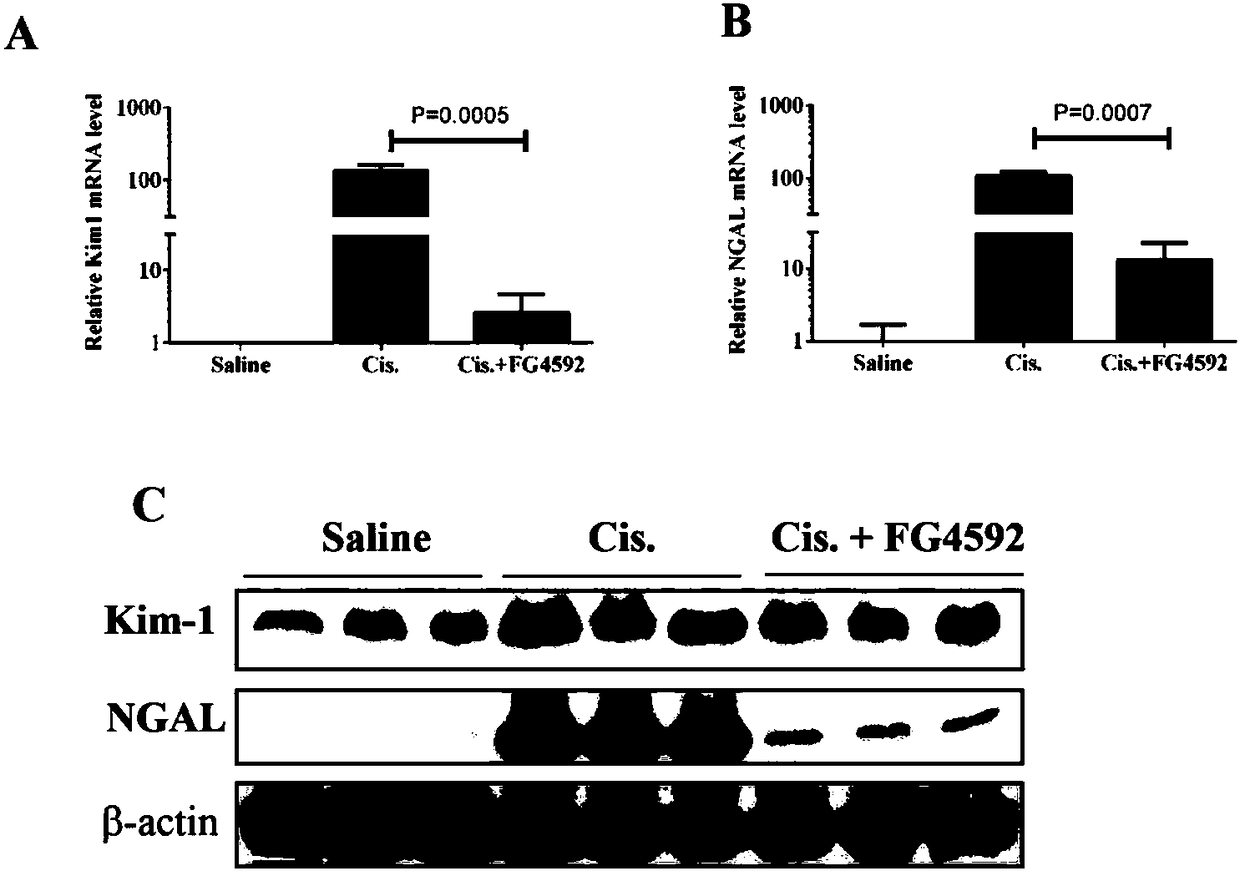
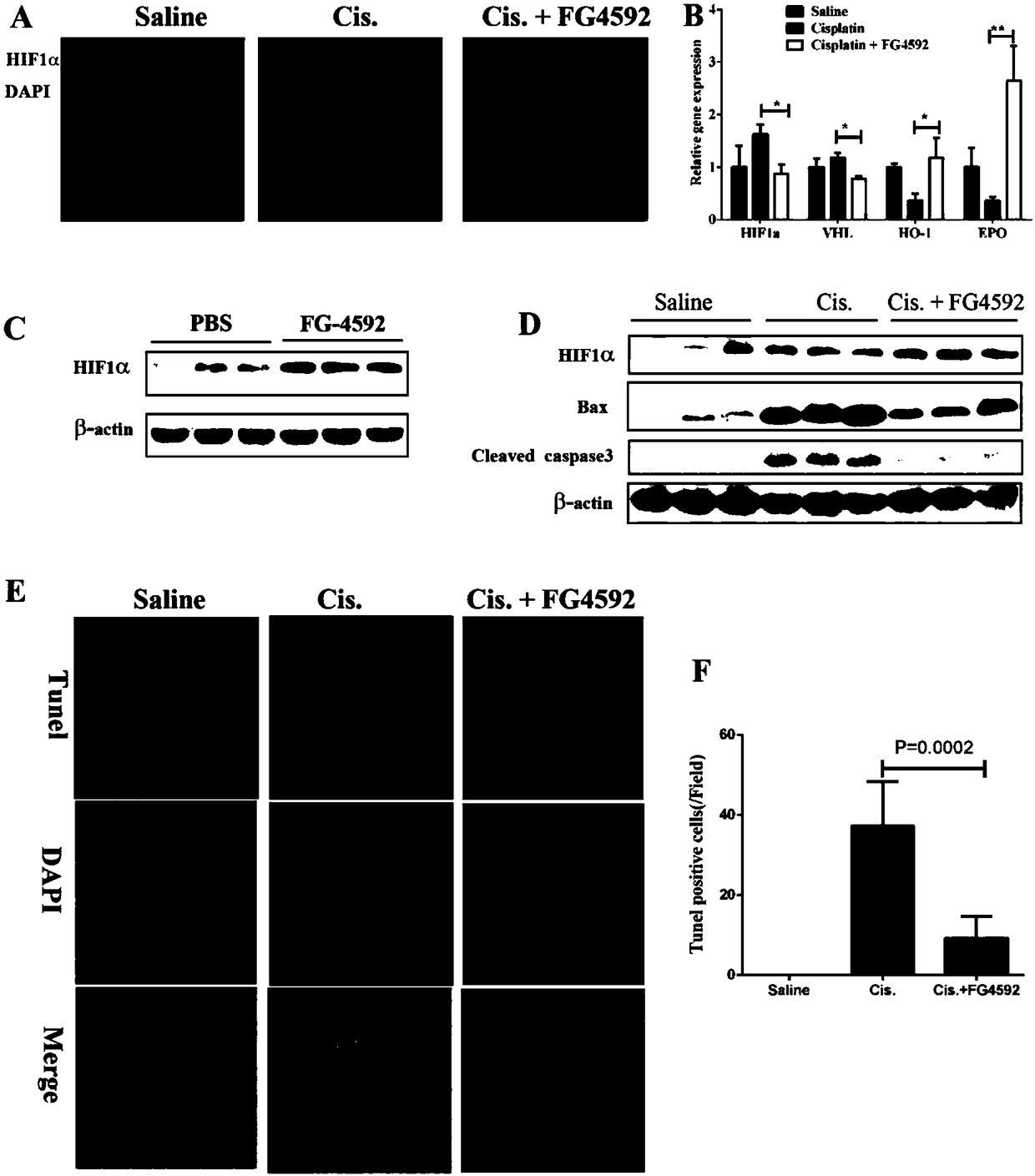
The invention discloses a use of a hypoxia-inducible factor prolyl hydroxylase activity inhibitor in the preparation of a drug for preventing and treating acute kidney injury. The HIF prolyl hydroxylase activity inhibitor is Roxadustat (FG-4592). The HIF prolyl hydroxylase activity inhibitor is an HIF family selective inhibitor, and can alleviate pathological damages of cisplatin-induced acute kidney injury to kidneys and improve the functions of the kidneys. The FG-4592 can reduce apoptosis of renal tubular cells and renal tubular functions, improve the pathological injury and renal functionsof the kidneys and protect the structures and the functions of the kidneys in the acute kidney injury by activating HIF1alpha, promoting the expression of EPO and HO-1, inhibit apoptosis and down-regulate the release of inflammatory factors. The FG-4592 is currently in a Phase III clinical research stage in the field of prevention and treatment of chronic renal anemia, and it is found that the FG-4592 is highly possible to provide the effective clinical drug for preventing and treating the AKI.
Owner:NANJING CHILDRENS HOSPITAL
Therapeutic agent for ischemia which inhibits apoptosis under ischemic condition
The present invention relates to a therapeutic agent for ischemia which inhibits apoptosis under ischemic condition. The therapeutic agent of present invention comprises antibacterial agents of quinolones, quinones, aminoglycosides or chlorampheni-col as an active ingredient. Since the invented therapeutic agent improved the viability of cells under hypoxic and hypoglycemic condition, it can be clinically for ischemic diseases such as applied as a potentiad drug for ischemia-associated infarction and cerebral infarction.
Owner:HYPOXICO
Glucose oxidase compositions as a neonate anticonvulsant
ActiveUS20200345817A1Improve clinical efficacyNervous disorderPeptide/protein ingredientsAntiepileptic drugGluconic acid
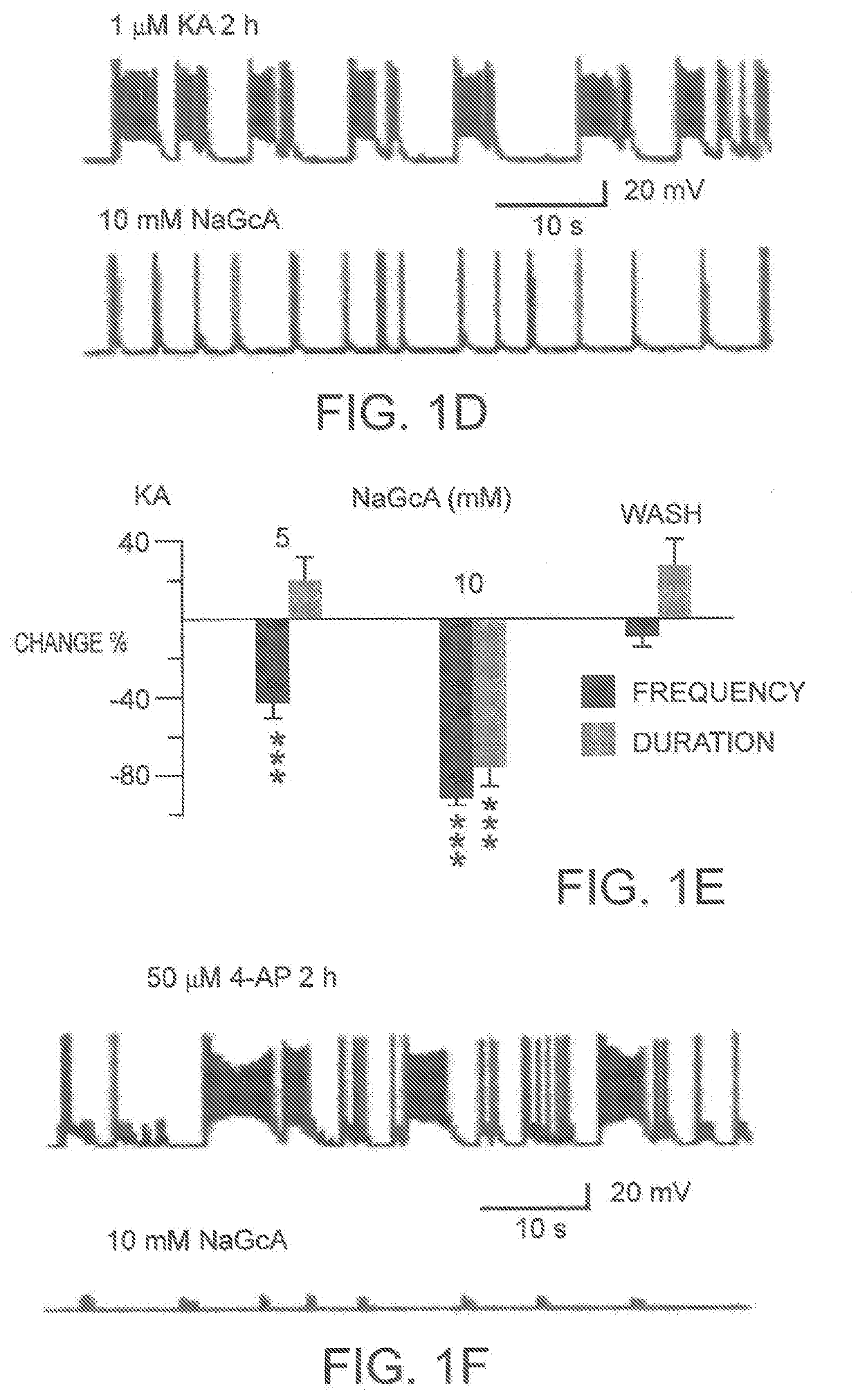
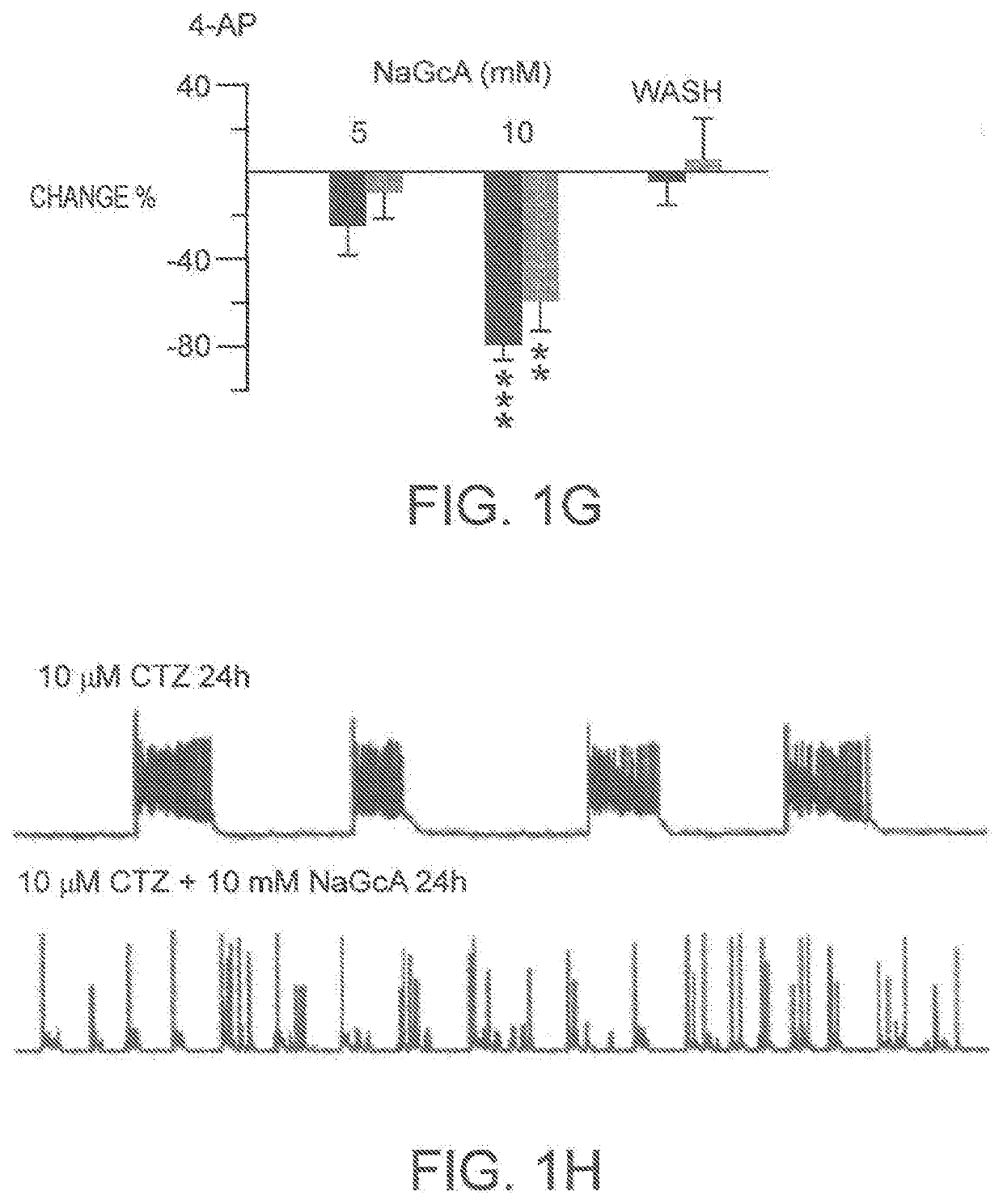
Neonatal seizure is different from adult seizure, and many anti epileptic drugs that are effective in adults often fail to treat neonatal seizure. Gluconic acid, a natural organic acid enriched in fruits and honey, and the glucose oxidase enzyme, is shown herein to potently inhibit neonatal epilepsy both in vitro and in vivo. Sodium gluconate is shown to inhibit epileptiform burst activity in cell cultures and protect neurons from kainic acid-induced cell death. Sodium gluconate also inhibited epileptiform burst activity in brain slices in a manner that was much more potent in neonatal animals than in older animals. Consistently, in vivo EEC recordings also revealed that sodium gluconate inhibited the epileptic seizure activity in a manner that was much more potent in neonates than in adult animals. Mechanistically, sodium gluconate inhibits voltage-dependent CLC-3 C1− channels both in neuronal cultures and in hippocampal slices. Together, these data suggest a novel antiepileptic drug gluconate that potently inhibits neonatal seizures through blocking CLC-3 C1− channels.
Owner:PENN STATE RES FOUND
Application of p53 gene in reversing the malignant characteristics of oral mucosal malignant melanoma cells and its in vitro evaluation method
InactiveCN104862369BInhibit growthComprehensive detectionMicrobiological testing/measurementIndividual particle analysisMelanomaWild type
The invention discloses the application of the p53 gene to reversing of mouth mucosa malignant melanoma cell malignant characteristics, and an in vitro evaluation method for reversing of the mouth mucosa malignant melanoma cell malignant characteristics, and belongs to the technical field of cellular and molecular biology. According to the invention, a gene therapy method is applied to the reversing of mouth mucosa malignant melanoma cell malignant characteristics and the in vitro evaluation method for reversing of the mouth mucosa malignant melanoma cell malignant characteristics; by introducing the exogenous wild-type p53 gene into a malignant melanoma-related cell strain, using various mechanisms, and depending on synthetic action of various signal transduction pathways, cell growth is inhibited, meanwhile, the cell cycle distribution is changed, cells are induced for apoptosis, and the malignant characteristics of cell transfer and invasion can be reversed. The evaluation method is simple and reliable, is scientific and rational, is capable of comprehensively detecting the reversing condition of the mouth mucosa malignant melanoma cell malignant characteristics, and can be used not only for studying of oral mucosa malignant melanoma pathogenesis, but also for screening of related drugs for oral mucosa malignant melanoma.
Owner:李龙江
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com