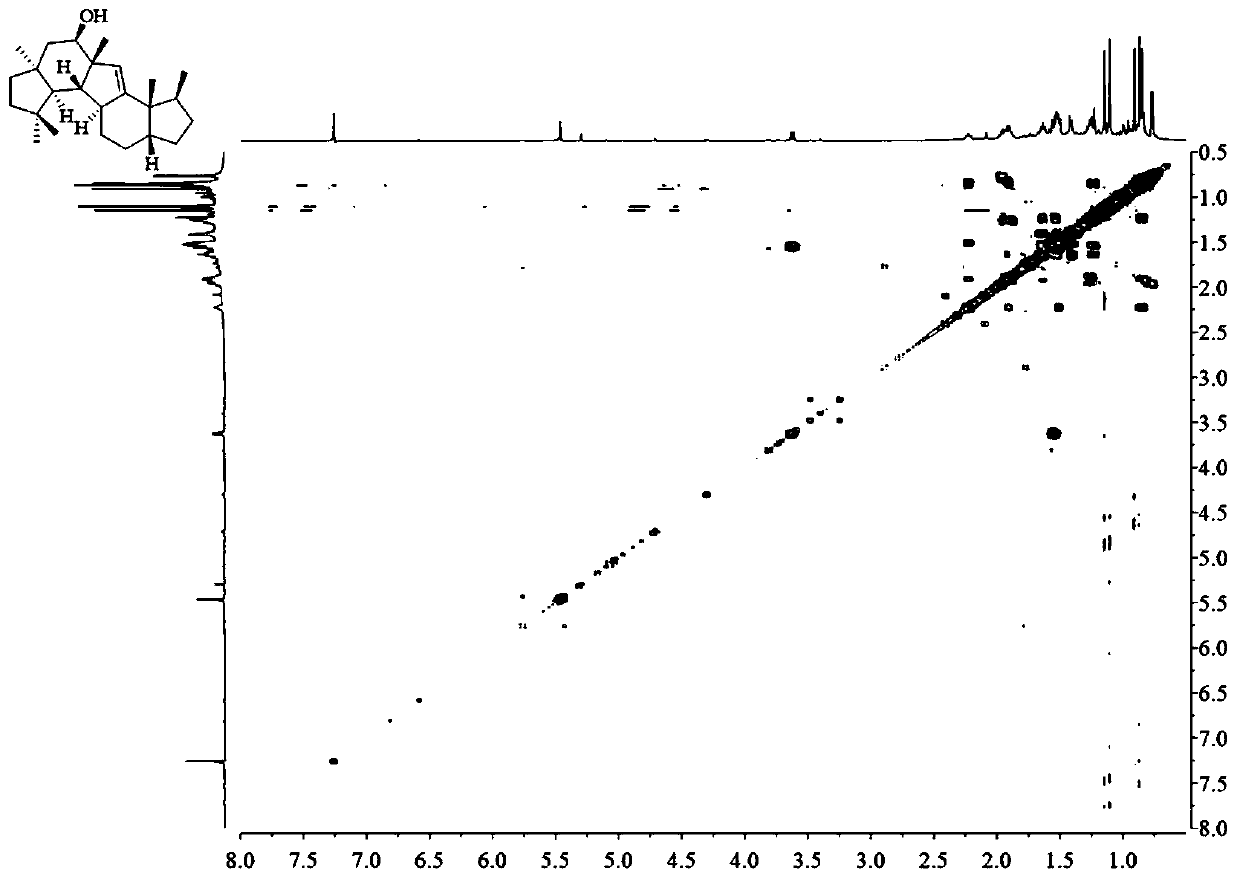
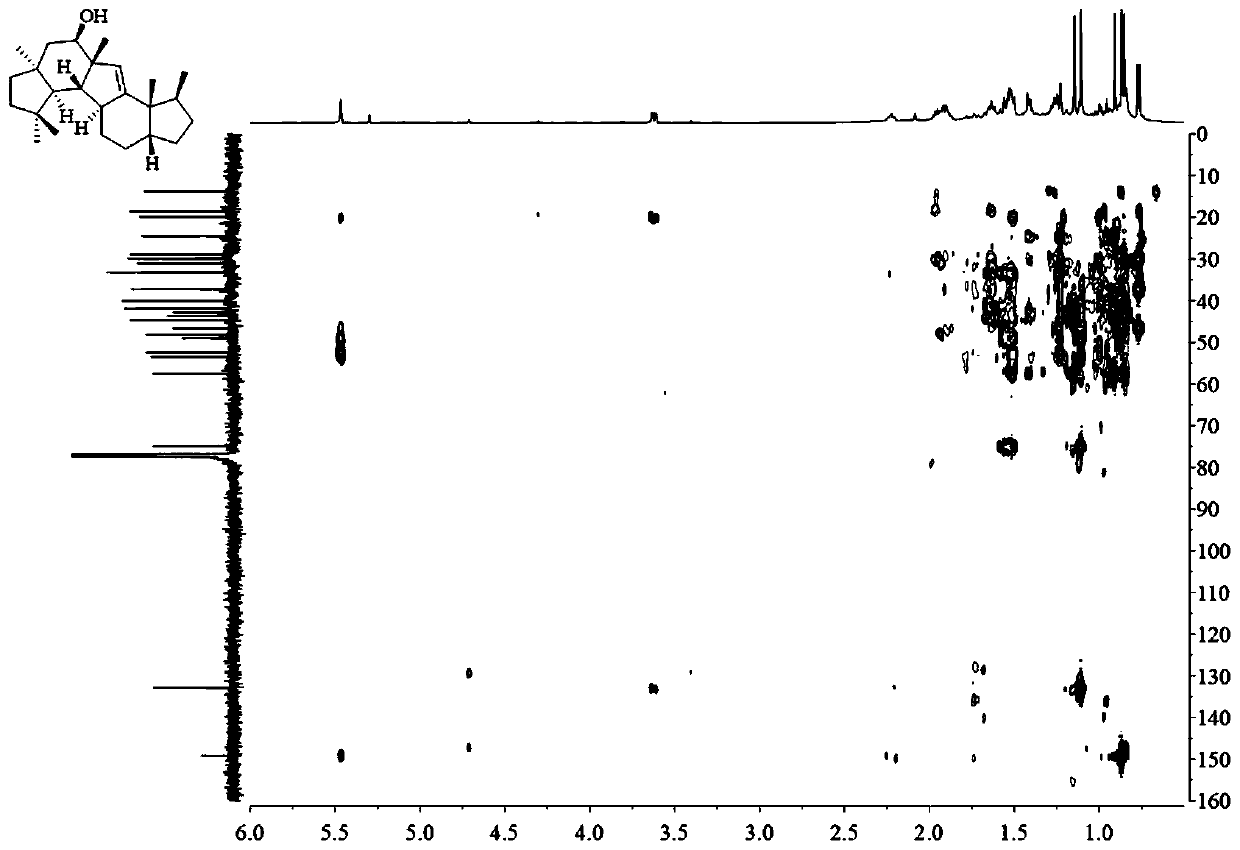
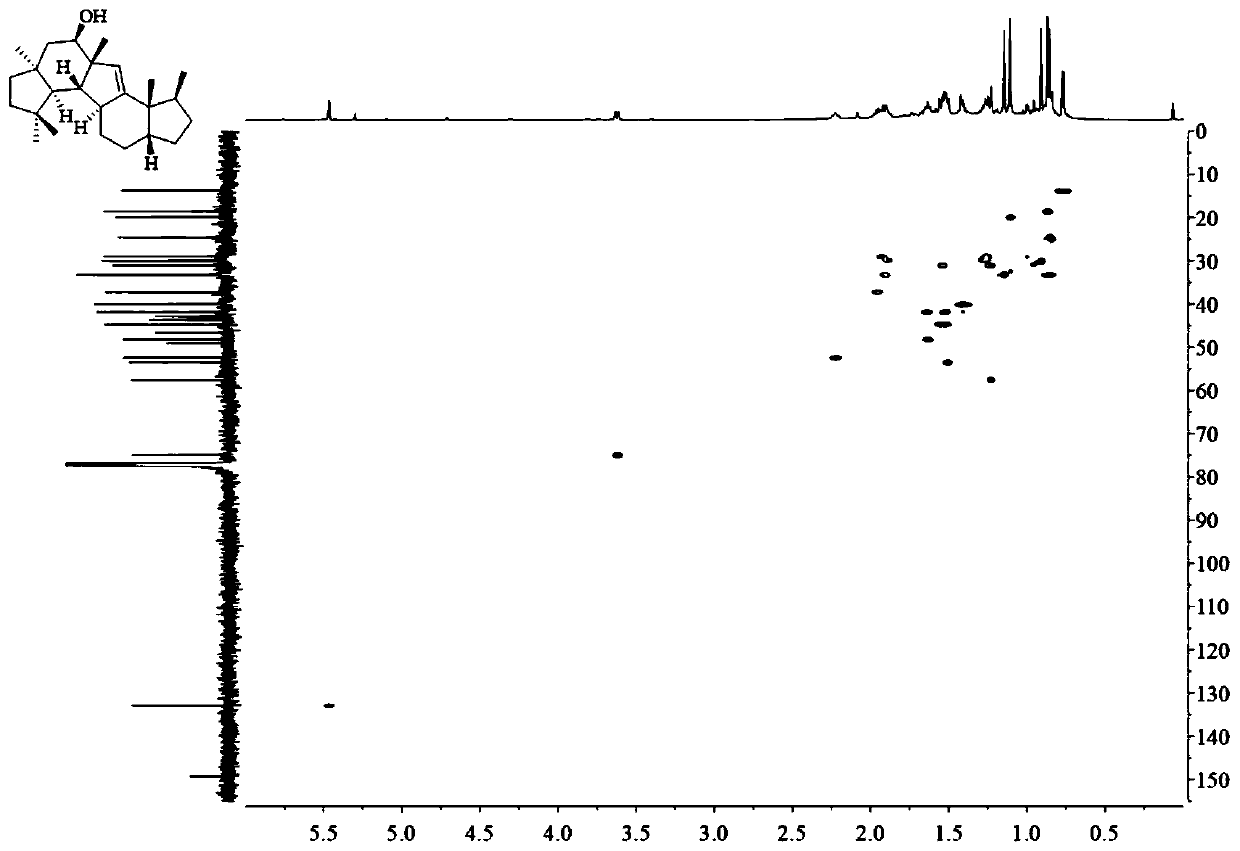
A kind of sesquiterpene compound peniroquesines and its preparation method and application
A technology of sesquiterpenes and compounds, which is applied in the field of sesquiterpenoids, can solve the problems of non-modularization of terpenoids, no synthetic strategy, and incomplete elucidation, and achieve strong stereoselectivity and simple and easy preparation methods line, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The present invention also provides the preparation method of the sesquiterpene compound peniroquesines described in the above technical scheme, comprising the following steps:
[0061] (1) Inoculate Penicillium loudii into the fermentation medium and ferment to obtain the fermentation product of Penicillium loudii; the preservation number of the Penicillium loudii is CGMCC NO.14140;
[0062] (2) Ultrasonic extraction after mixing the fermented product of Penicillium loudii obtained in the step (1) with the alcohol solution to obtain the crude extract of peniroquesines;
[0063] (3) Using multiple groups of chloroform-methanol solutions with a volume ratio in the range of 100:0 to 20:1 as the eluting solvent, the crude extract of peniroquesines obtained in the step (2) was eluted by silica gel column chromatography, and the obtained multiple groups The eluent was purified by silica gel column chromatography to obtain the sesquiterpene compound peniroquesines,
[0064] ...
Embodiment 1
[0102] Preparation of sesquiterpenoids peniroquesines B, C, D, H and J
[0103] 1. Strain activation: Inoculate Penicillium loudii (preservation number CGMCC NO.14140) on PDA slant medium, culture at 28°C for 3-7 days, and store in a 4°C refrigerator for later use.
[0104] 2. Preparation of fermentation medium: take the washed potatoes, divide them into potato pieces with a diameter of 1cm, put them in a tissue culture bottle, 50g / bottle, cover the tissue culture bottle, sterilize at 120°C for 30 minutes, and cool to obtain Fermentation medium.
[0105] 3, the Penicillium loudii activated in step 1 is inoculated to the fermentation medium prepared in step 2 according to an inoculum size of 1%, and then cultured at a constant temperature at 28°C for 30d after adding a cover to obtain the Penicillium loudii fermentation product.
[0106] 4. Get 50g of the Penicillium roquesine ferment obtained in step 3 and mix it with 100mL of methanol. The mixed solution is ultrasonicated fo...
Embodiment 2
[0124] Anti-inflammatory activity of Example 2 compounds peniroquesines B, C, D, H and J:
[0125] Nitric oxide (NO) has extensive and important biological regulation functions, and plays an important role in inflammation, tumor and cardiovascular system. When immune cells are stimulated by microbial endotoxins and inflammatory mediators, they will generate a large amount of inducible nitric oxide synthase (iNOS) and produce NO for immune response. Therefore, inhibition of NO production is a direct indicator of the anti-inflammatory activity of the compound.
[0126] (1) Take the mouse mononuclear macrophage RAW264.7 in the logarithmic growth phase (purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences), induce stimulation with 1 μg / ml LPS, and add the compounds peniroquesines B and C prepared in Example 1 at the same time , D, H and J treatments, the final concentrations of compound peniroquesines B, C, D, H and J were 3.125 μM, 6.25 μM, 12.5 μM, 25 μM and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com