Preparation method of amide compounds
An amide compound, organic amine technology, applied in the field of organic chemistry or pharmaceutical intermediates, can solve the problems of difficult separation of by-products, narrow substrate adaptability, incomplete reaction, etc. mild effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
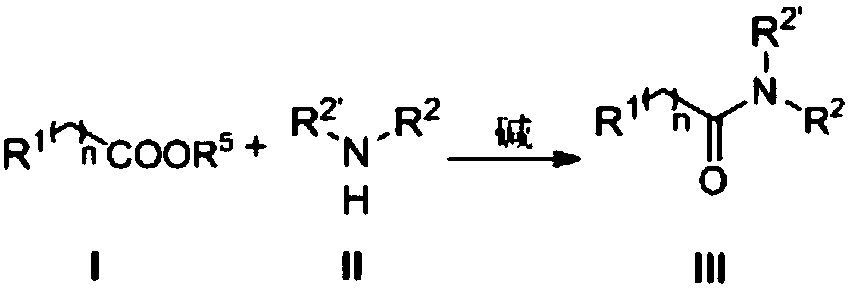
[0029] A kind of preparation method of amides compound, reaction equation is as follows:
[0030]
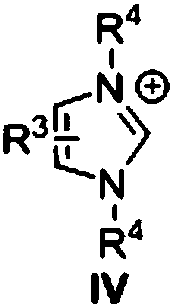
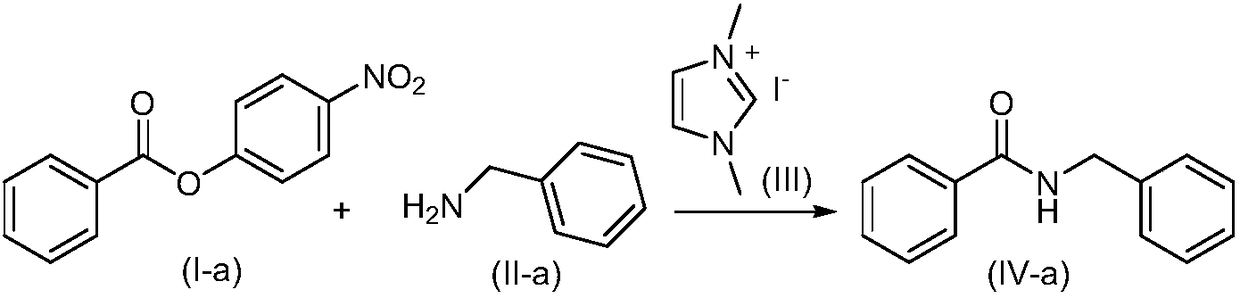
[0031] Under nitrogen atmosphere, nitrogen heterocyclic carbene III (33.6mg, 0.15mmol) was dissolved in tetrahydrofuran (2.0mL), DBU (22.8mg, 0.15mmol) and benzoate I-a (121.61mg, 0.5mmol) were added under stirring , the reaction system dropped to zero, slowly added benzylamine II-a (64.3mg, 0.6mmol) dropwise, stirred at room temperature for 15min, after the reaction solution was concentrated, extracted with dichloromethane 3 times, washed with saturated brine, dried over anhydrous sodium sulfate, The crude product was purified by column chromatography to obtain 98.2 mg of pure product (IV-a), with a yield of 93%. The NMR characterization data are as follows: 1 H-NMR (400M, CDCl 3 ):7.79-7.77(m,2H),7.5-7.46(m,1H),7.42-7.38(m,2H),7.34-7.25(m,5H),6.65(s,1H),4.61(d,J =8.0Hz, 1H).
Embodiment 2
[0033] A kind of preparation method of amides compound, reaction equation is as follows:
[0034]
[0035] Under nitrogen atmosphere, nitrogen heterocyclic carbene III (33.6mg, 0.15mmol) was dissolved in tetrahydrofuran (2.0mL), DBU (22.8mg, 0.15mmol), benzoate I-b (136.62mg, 0.5mmol) were added under stirring , the reaction system dropped to zero, slowly dropwise added benzylamine II-a (64.3mg, 0.6mmol), stirred at room temperature for 15min, after the reaction solution was concentrated, extracted with dichloromethane 3 times, washed with saturated brine, dried over anhydrous sodium sulfate, The crude product was purified by column chromatography to obtain 112.2 mg of pure product (IV-b), with a yield of 93%. The NMR characterization data are as follows: 1 H NMR (400MHz, CDCl 3 )δ7.75(d, J=9.6Hz, 2H), 7.35-7.26(m, 5H), 6.90(d, J=9.2Hz, 2H), 6.39(s, 1H), 4.62(d, J=5.6 Hz, 2H), 3.84(s, 3H).
Embodiment 3
[0037] A kind of preparation method of amides compound, reaction equation is as follows:
[0038]
[0039] Under nitrogen atmosphere, nitrogen heterocyclic carbene III (33.6mg, 0.15mmol) was dissolved in tetrahydrofuran (2.0mL), DBU (22.8mg, 0.15mmol), benzoate I-c (43.2mg, 0.5mmol) were added under stirring , the reaction system dropped to zero, slowly dropwise added benzylamine II-a (64.3mg, 0.6mmol), stirred at room temperature for 15min, after the reaction solution was concentrated, extracted with dichloromethane 3 times, washed with saturated brine, dried over anhydrous sodium sulfate, The crude product was purified by column chromatography to obtain 120.4 mg of pure product (IV-c), with a yield of 94%. The NMR characterization data are as follows: 1 H NMR (400MHz, CDCl 3 ) 8.02 (d, J = 8.8Hz, 1H), 7.66-7.26 (m, 9H), 6.28 (bs, 1H), 4.60 (d, J = 6.4Hz, 2H).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap