CQD (Carbon Quantum Dot) adjuvant and vaccine containing same
A technology for carbon quantum dots and vaccines, applied in vaccines, veterinary vaccines, medical preparations containing active ingredients, etc., can solve problems such as unfavorable injection, limited combination methods, poor emulsification, etc. Long-lasting immune memory and effective immune protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Preparation of carbon quantum dots
[0029] Humic acid (0.2 g) was dissolved in 50 mL of secondary water and transferred to an autoclave with Teflon. The autoclave was heated at 180°C for 5 hours and cooled naturally to obtain a dark brown solution. The reaction solution was centrifuged at 3000 rpm for 15 minutes. Subsequently, the precipitate was discarded, and the supernatant was dialyzed in a dialysis bag (MWCO=1000) for 48 hours to remove small molecule impurities. Afterwards, the supernatant was centrifuged at 12000 rpm for 15 minutes, and the obtained fluorescent CQDs were dried in a vacuum oven and stored in a refrigerator at 4 °C.
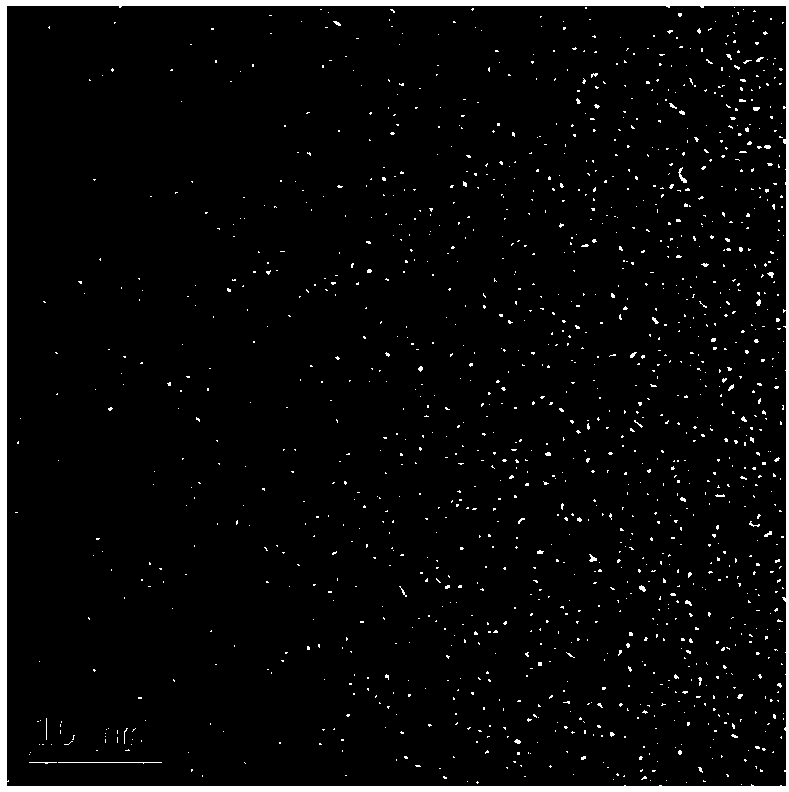
[0030] The size and morphology of the synthesized CQDs were studied by transmission electron microscopy (TEM), as figure 1 shown. Depend on figure 1 It can be seen that the CQDs particles prepared in this example are spherical with a diameter of 3-5 nm.
Embodiment 2
[0031] Embodiment 2: Expression and identification of ALV-J gp85 protein
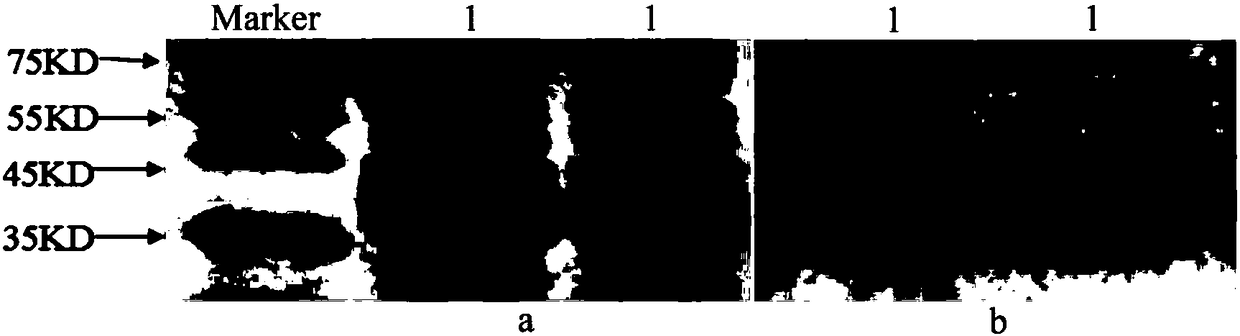
[0032] Firstly, the recombinant plasmid (PET28a-gp85) was transformed into Rosetta (DE3) cells, and then induced with 0.5mM IPTG for 6 hours at 37°C to obtain the recombinant gp85 protein. The recombinant gp85 protein was purified by high-affinity Ni-NTA column and identified by sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) after dialysis ( figure 2 a). Proteins were subjected to gp85-specific M antibody JE9 under Western blot analysis ( figure 2 b). The concentration of gp85 protein collected by PEG6000 was determined to be 1.2 mg / mL by thin layer chromatography.
Embodiment 3
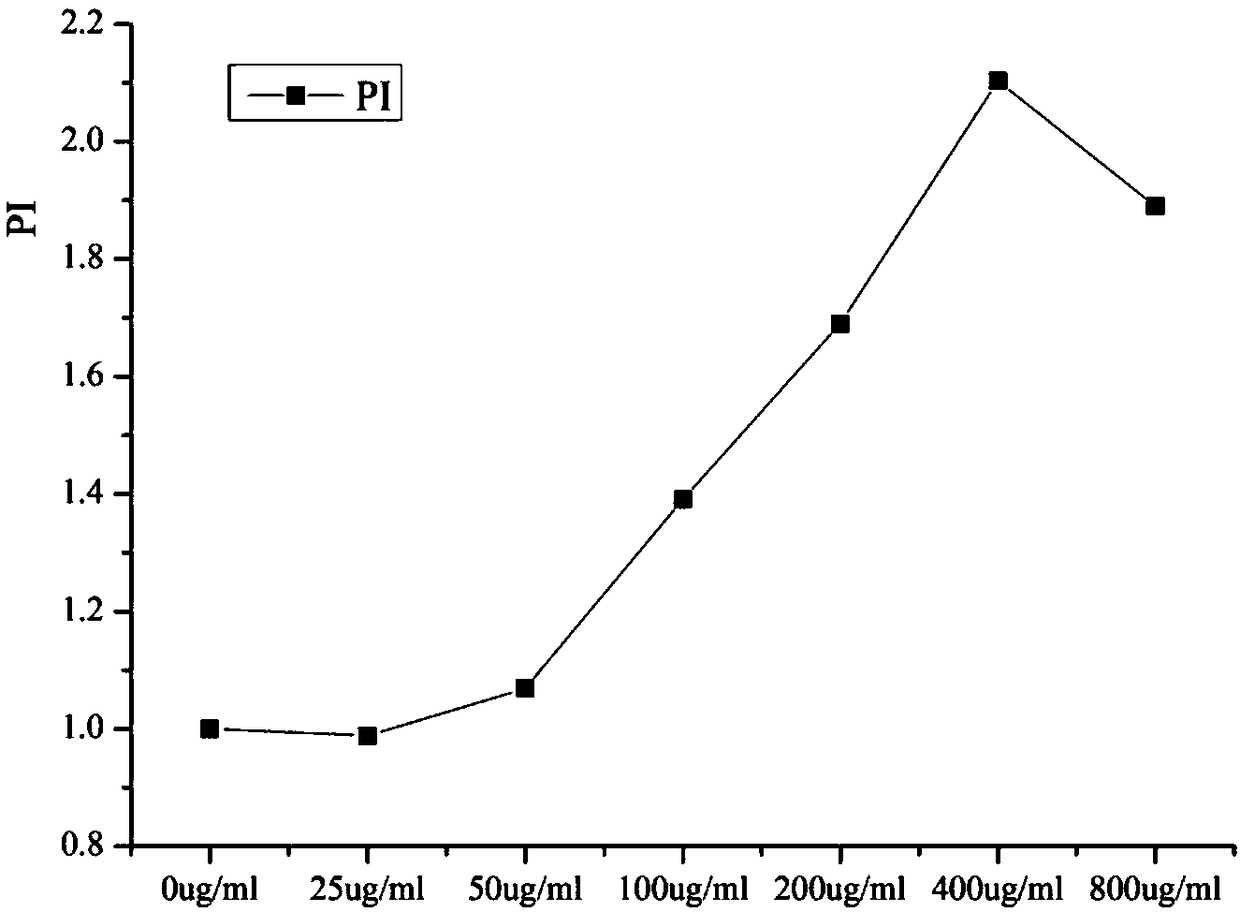
[0033] Example 3: Effect of carbon quantum dots on the activity of chicken lymphocytes
[0034] MTT assay was used to determine the effect of CQDs on the viability of chicken lymphocytes. details as follows:
[0035] Chicken lymphocytes were grown in 96-well microplates in Roswell Park Memorial Institute medium containing 10% fetal bovine serum and 1% penicillin / streptomycin in 5% CO 2 Incubate at 37°C for 24 hours. Solutions of synthetic CQDs (prepared in Example 1) at different concentrations were loaded into each well; each concentration was prepared in triplicate and incubated for 24 hours. Afterwards, 20 mL of 5 mg / mL 3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide (MTT) solution was added to each well containing cells and incubated Incubate for 4 hours. The medium was removed and 150 mL of DMSO was added to release the methyl groups. The mixture was shaken for about 15 minutes, and the luminosity (OD) was measured by a microplate reader. Cell viability w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com