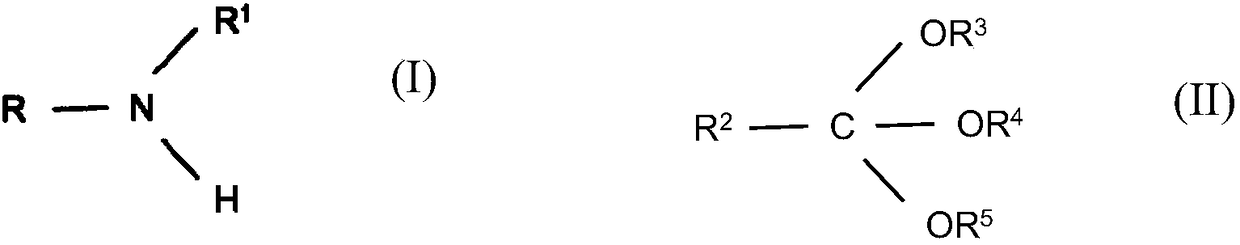
Reductive alkylation of amines with orthocarboxylic acid esters
A carboxylate, alkylation technology, applied in reductive alkylation preparation, organic chemistry, cyanide reaction preparation, etc., can solve problems such as difficult separation of amino acids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0099] General procedure for the reductive alkylation of amines with orthocarboxylates
[0100] Fill the autoclave with catalyst (1 mol%, based on the molar amount of amine), flush with argon and add amine (0.1 mol) and orthocarboxylate (0.11-0.3 mol) in 10 ml of methanol (or ethanol) solution and 0.5ml of 0.2M anhydrous p-toluenesulfonic acid in methanol (or ethanol). The mixture was heated to 120° C. and injected with hydrogen to 40 bar, then the mixture was stirred at constant pressure until no more hydrogen uptake could be detected (0.2-6 hours). After filtering off the catalyst, the filtrate was distilled.
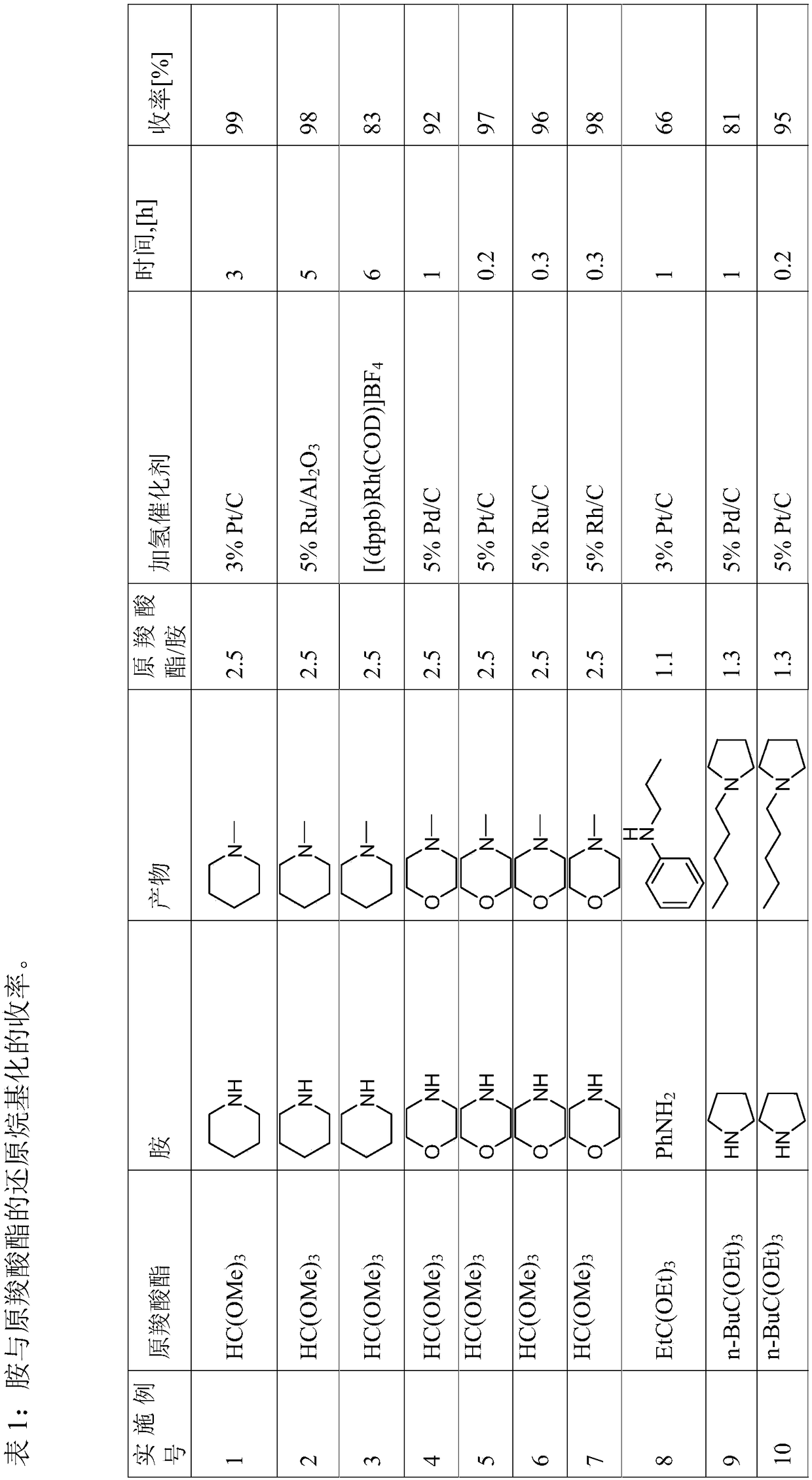
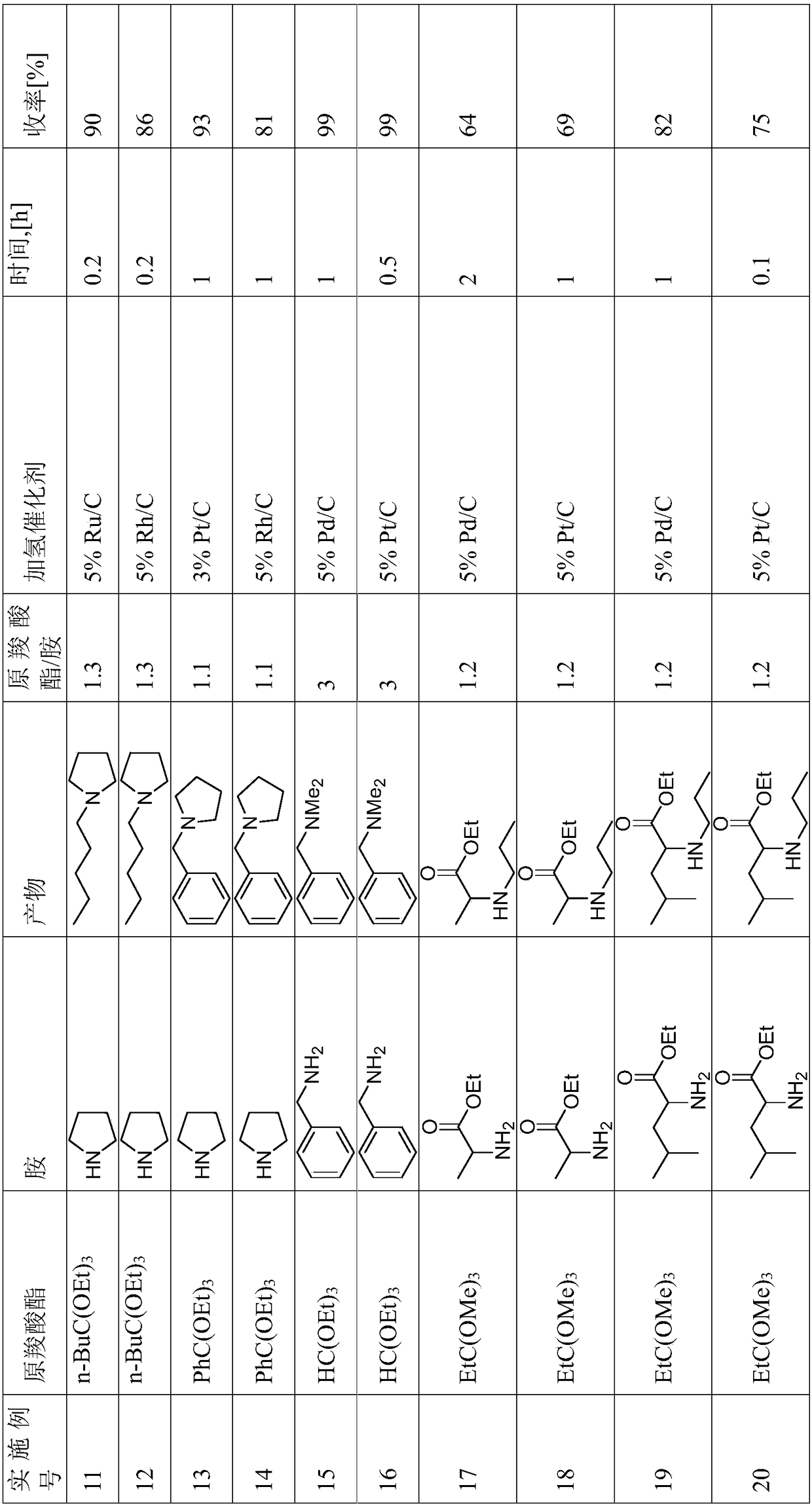
[0101] Yields can be found in Table 1.
[0102]
[0103]
[0104]
[0105]
[0106]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com