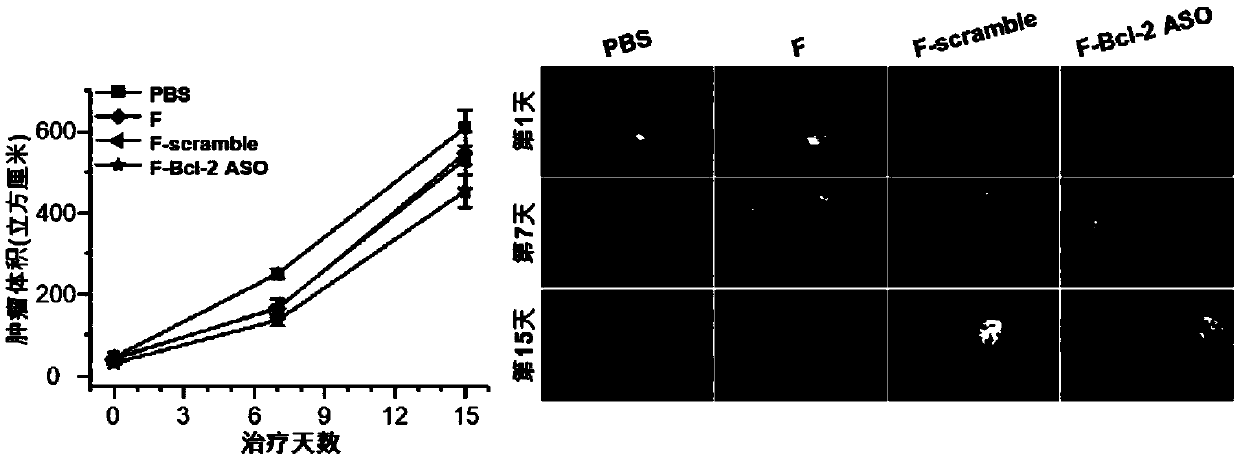
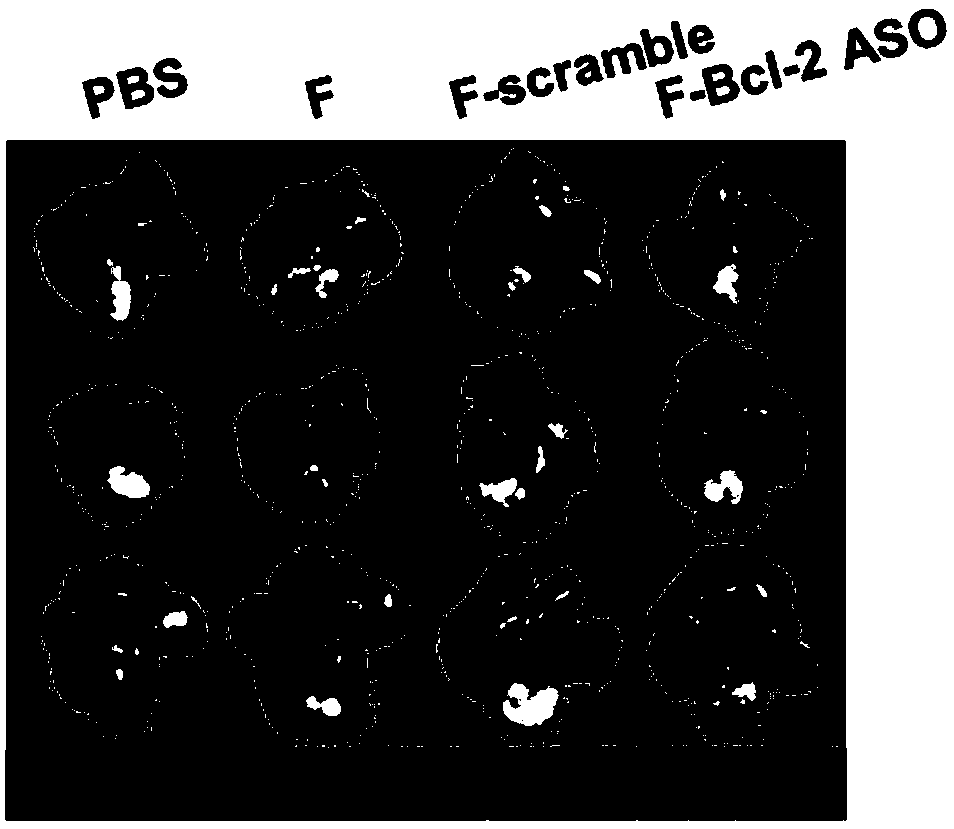
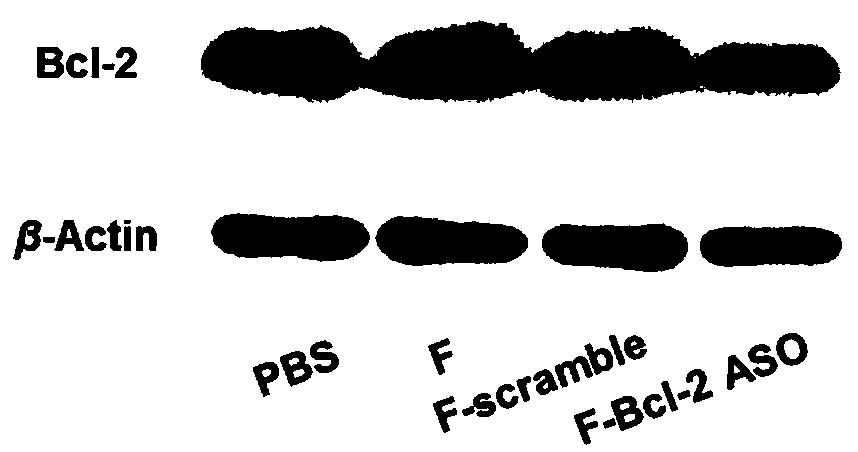
Functional nucleic acid of skeleton integrated nucleoside analog medicine as well as derivative and application thereof
A nucleoside analog and functional technology, applied in the field of biomedicine, can solve problems such as large differences in hydrophilicity and hydrophobicity, restrictions on the application of gene-chemotherapy combined therapy, and the difficulty in precise control of the release time sequence of gene drugs and chemotherapy drugs, etc., to achieve high efficiency Packed Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1 Bcl-2 antisense nucleotide (F-Bcl-2ASO) with integrated floxuridine backbone
[0077] 1.1 Backbone-integrated Bcl-2 antisense nucleotides of floxuridine (F-Bcl-2 ASO) Synthesis
[0078] During DNA solid-phase synthesis, all nucleotides T in the antisense nucleotides were replaced with the antineoplastic drug floxuridine (F) in this embodiment. specifically,
[0079] Place the phosphoramidite monomer and DNA phosphoramidite monomer of floxuridine drug in the corresponding position of the DNA solid-phase synthesizer, add ordinary microporous glass beads (CPG) into the reaction column, and input 5'-CAG CGF GCG CCA FCC FFC CC AFCCFCCFCC-3'sequence information, add catalyst, capping, oxidation and deprotection reagents, synthesize fluorine-containing uridine sequence, and then obtain F- Bcl-2 ASO sequence.
[0080] In addition, according to the same method, enter the sequence of 5'-AAF ACF CCG AAC GFG FCA CGFCCFCCF CAC-3' into the solid-phase synthesizer to sy...
Embodiment 2
[0089] Example 2 Bcl-2 / xL antisense nucleotides (F-Bcl-2 / xLASO) with backbone integration of floxuridine
[0090] 2.1 Backbone-integrated Bcl-2 / xL antisense nucleotides of floxuridine (F-Bcl-2 / xL ASO) Synthesis
[0091] During DNA solid-phase synthesis, all nucleotides T in the antisense nucleotides were replaced with the antineoplastic drug floxuridine (F) in this embodiment.
[0092] Specifically, the phosphoramidite monomer and DNA phosphoramidite monomer of floxuridine drug are placed in the corresponding positions of the DNA solid-phase synthesizer, and the corresponding common microporous glass beads (CPG) are added to the reaction column, and the input 5' -AAG GCA FCC CAG CCF CCGFF CCFCCFCCF A-3'sequence information, adding catalyst, capping, oxidation and deprotection reagents, after synthesizing fluorine-containing uridine sequence, ammonolysis, nitrogen blowing, preparative chromatography separation and purification, deprotection and concentration The F-Bcl-2 / xL A...
Embodiment 3
[0096] Example 3 Spherical nucleic acid SNA (F-Bcl-2ASO) constructed by Bcl-2 antisense nucleotides with integrated backbone of floxuridine
[0097] 3.1 Bcl-2 antisense nucleotide with floxuridine integrated into DBCO modified backbone (F-Bcl-2 ASO-DBCO) Synthesis
[0098] When preparing the backbone-integrated Bcl-2 antisense nucleotides of floxuridine by solid-phase synthesis, amino-modified microporous glass beads (NH 2 -CPG), the Bcl-2 antisense nucleotide (F-Bcl-2ASO-NH 2 ).
[0099] Add 200 equivalents of DBCO-NHS ester to the above-mentioned antisense nucleotide sequence in DMSO mixed solution containing 30% phosphate buffer, and react at room temperature for 24 hours to obtain the Bcl-2 antisense with DBCO-modified backbone integrating floxuridine. Sense nucleotide (F-Bcl-2ASO-DBCO) ( Figure 5 ).
[0100] The above crude product was purified by multiple extractions with ethyl acetate, ethanol precipitation, centrifugation and other steps.
[0101] Finally, F-Bc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com