Pyrazole compounds and application thereof
A compound and pyrazole technology, applied in the field of pyrazole compounds, can solve the problems of many adverse reactions, drug resistance, limiting effect, etc., and achieve the effects of strong inhibitory activity, improved affinity and inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
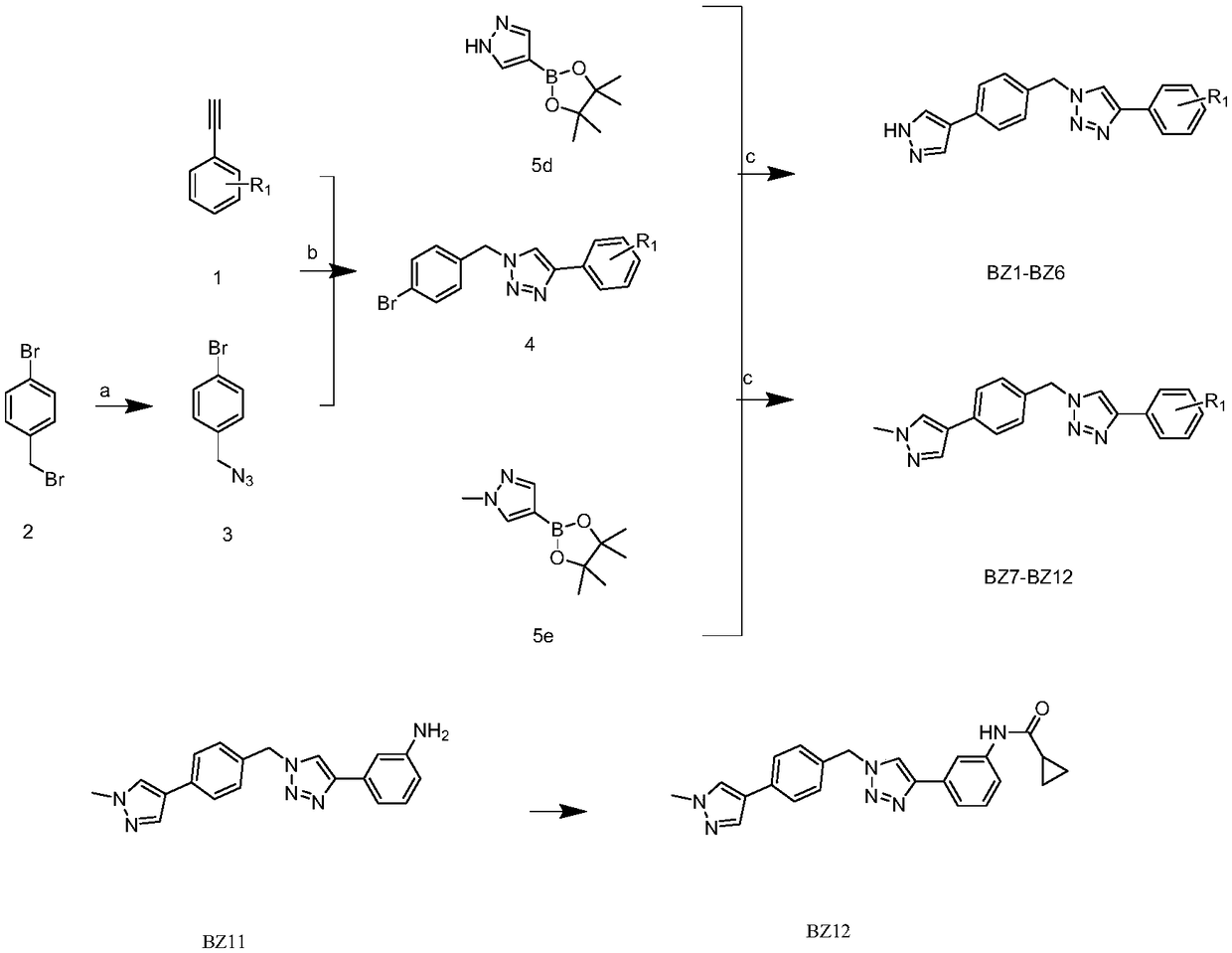
[0024] The present invention will be described in detail below in conjunction with the accompanying drawings.
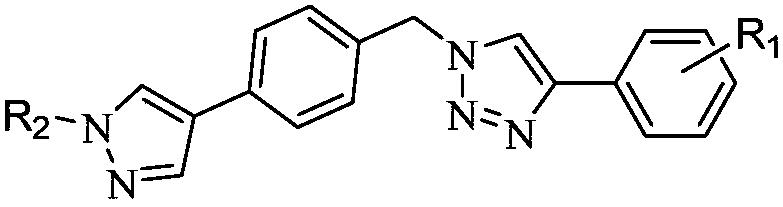
[0025] see figure 1 , the structural formula of the pyrazole compound of the present invention is:
[0026]
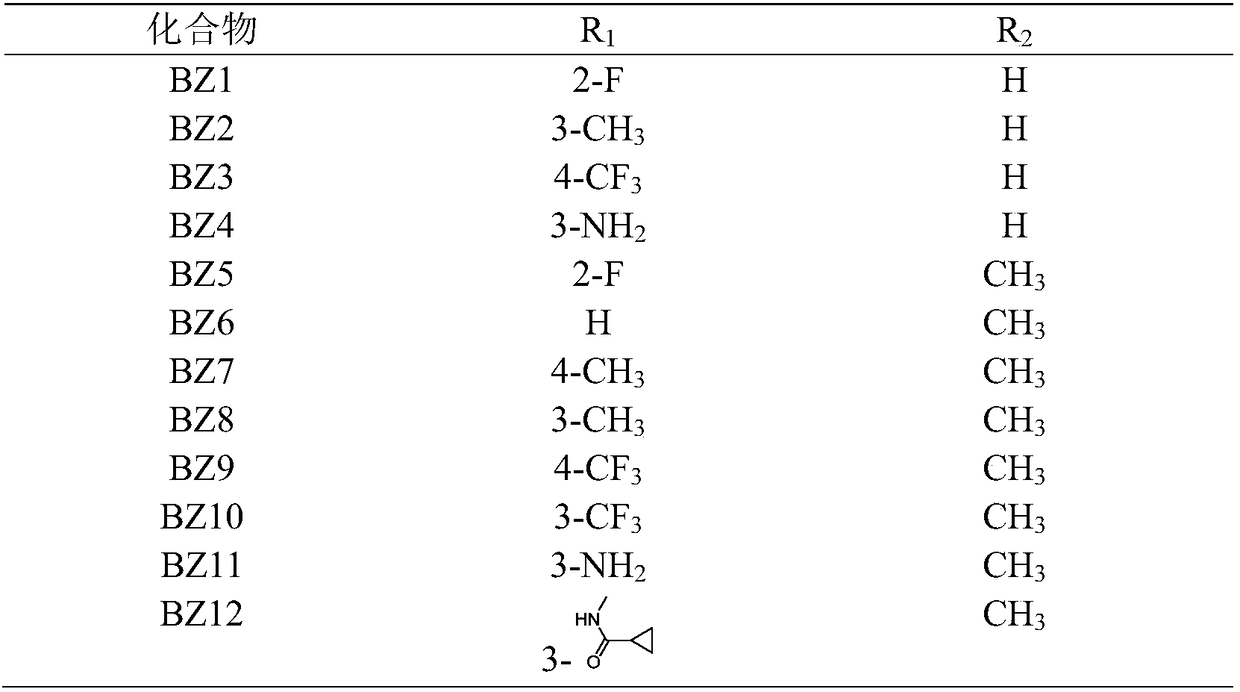
[0027] Among them, R 1 -F, -CH 3 、-CF 3 , -NH 2 , -F, H or R 2 -H or -CH 3 , see Table 1 for details.
[0028] The specific structure of the compound of the present invention in table 1
[0029]
[0030] R in Table 1 1 The numbers in represent R 1 The position of the group on the benzene ring.
[0031] see figure 1 , the specific preparation process of the present invention is as follows:
[0032] The synthesis of compound p-bromobian azide (3): under ice-bath conditions, 2.00g (8.00mmol) of p-bromobian bromine (2) was dissolved in 20mL of anhydrous DMF, and the sodium azide dissolved in water was slowly added dropwise (the amount of sodium azide is 0.78g (11.99mmol)) solution, rises to room temperature and then slowly drips the solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com