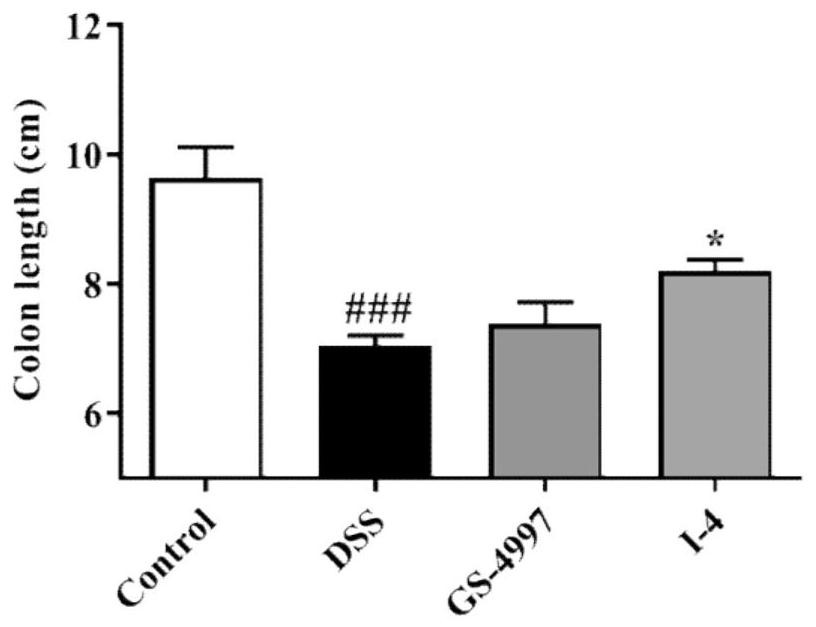
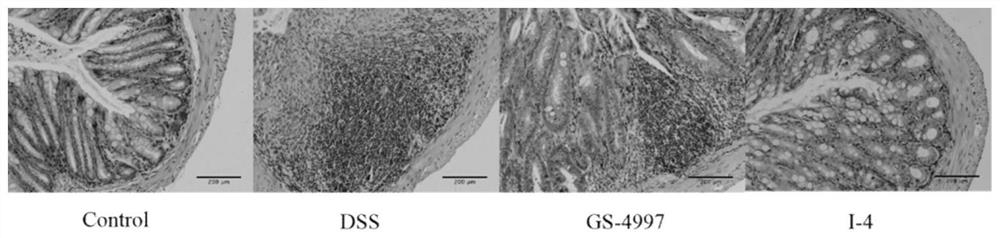
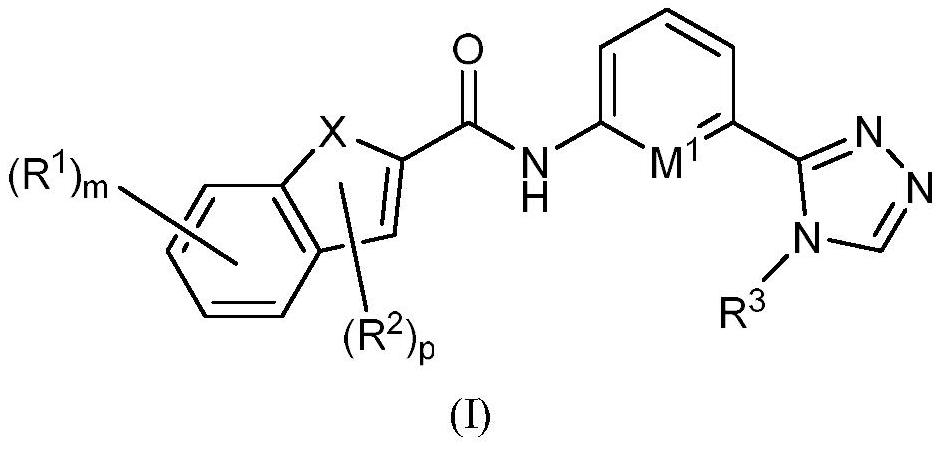
Indole ASK1 small-molecule inhibitor and preparation method and application thereof
A technology of small molecule inhibitors and indoles, which is applied in the field of indole ASK1 small molecule inhibitors and their preparation, and can solve problems such as single structure type
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of N-(6-(4-isopropyl-4H-1,2,4-triazol-3-yl)pyridin-2-yl)-1H-indole-2-carboxamide (I-1)
[0045]
[0046] The preparation of the first step 6-aminopyridinecarbohydrazide
[0047]
[0048] Methyl 6-aminopicolinate (5.0 g, 32.9 mmol) was dissolved in MeOH, and N 2 h 4 ·H 2 O (3.22g, 65.8mmol), the reaction solution was heated to reflux for 3h; cooled to room temperature, a solid precipitated out, the filter cake was washed with EA after suction filtration, and the target product (4.5g, 90%) was obtained after vacuum drying.
[0049] ESI-MS m / z:153.1[M+H] + .
[0050] Preparation of the second step 6-(4-isopropyl-4H-1,2,4-triazol-3-yl)pyridin-2-amine
[0051]
[0052] 6-Aminopyridinecarbohydrazide (4.5g, 29.61mmol) was dissolved with anhydrous Dioxane, triethyl orthoformate (5.92mL, 35.53mmol) was added, N 2 React under protection at 75°C for 1h. Add CH via syringe 3 COOH (5.08mL, 88.83mmol), cyclopropylamine (2.05mL, 29.61mmol), reacted at 110°...
Embodiment 2
[0060] 7-Chloro-N-(6-(4-isopropyl-4H-1,2,4-triazol-3-yl)pyridin-2-yl)-1H-indole-2-carboxamide (I- 2) Preparation
[0061]
[0062] Reference for the preparation of 7-chloro-N-(6-(4-isopropyl-4H-1,2,4-triazol-3-yl)pyridin-2-yl)-1H-indole-2-carboxamide Example 1.
[0063] 1 H NMR (300MHz, DMSO-d 6 )δ: 12.06(s, 1H), 10.85(s, 1H), 8.90(s, 1H), 8.26(dd, J=6.3, 0.3Hz, 1H), 8.06(t, J=8.7, 1H), 7.84 (dd, J=7.6,0.6Hz,1H), 7.68(d,J=6.0Hz,1H),7.55(d,J=2.1Hz,1H),7.37(dd,J=5.7,0.6Hz,1H) ,7.12(t,J=7.8Hz,1H),5.54-5.65(m,1H),1.48(d,J=5.1Hz,6H).
[0064] ESI-MS m / z:381.1.[M+H] + .
Embodiment 3
[0066] N-(6-(4-isopropyl-4H-1,2,4-triazol-3-yl)pyridin-2-yl)-6-methoxy-1H-indole-2-carboxamide ( I-3) Preparation
[0067]
[0068] N-(6-(4-isopropyl-4H-1,2,4-triazol-3-yl)pyridin-2-yl)-6-methoxy-1H-indole-2-carboxamide Preparation with reference to Example 1.
[0069] 1H NMR (300MHz, DMSO) δ11.70(s, 1H), 10.56(s, 1H), 8.88(s, 1H), 8.23(d, J=8.3Hz, 1H), 8.02(t, J=7.9Hz ,1H), 7.84(d, J=7.5Hz,1H), 7.63–7.47(m,2H), 6.93(s,1H), 6.75(d,J=8.7Hz,1H), 5.66-6.45(m, 1H), 3.80(s, 3H), 1.47(d, J=6.5Hz, 6H).
[0070] ESI-MS m / z:377.2[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com