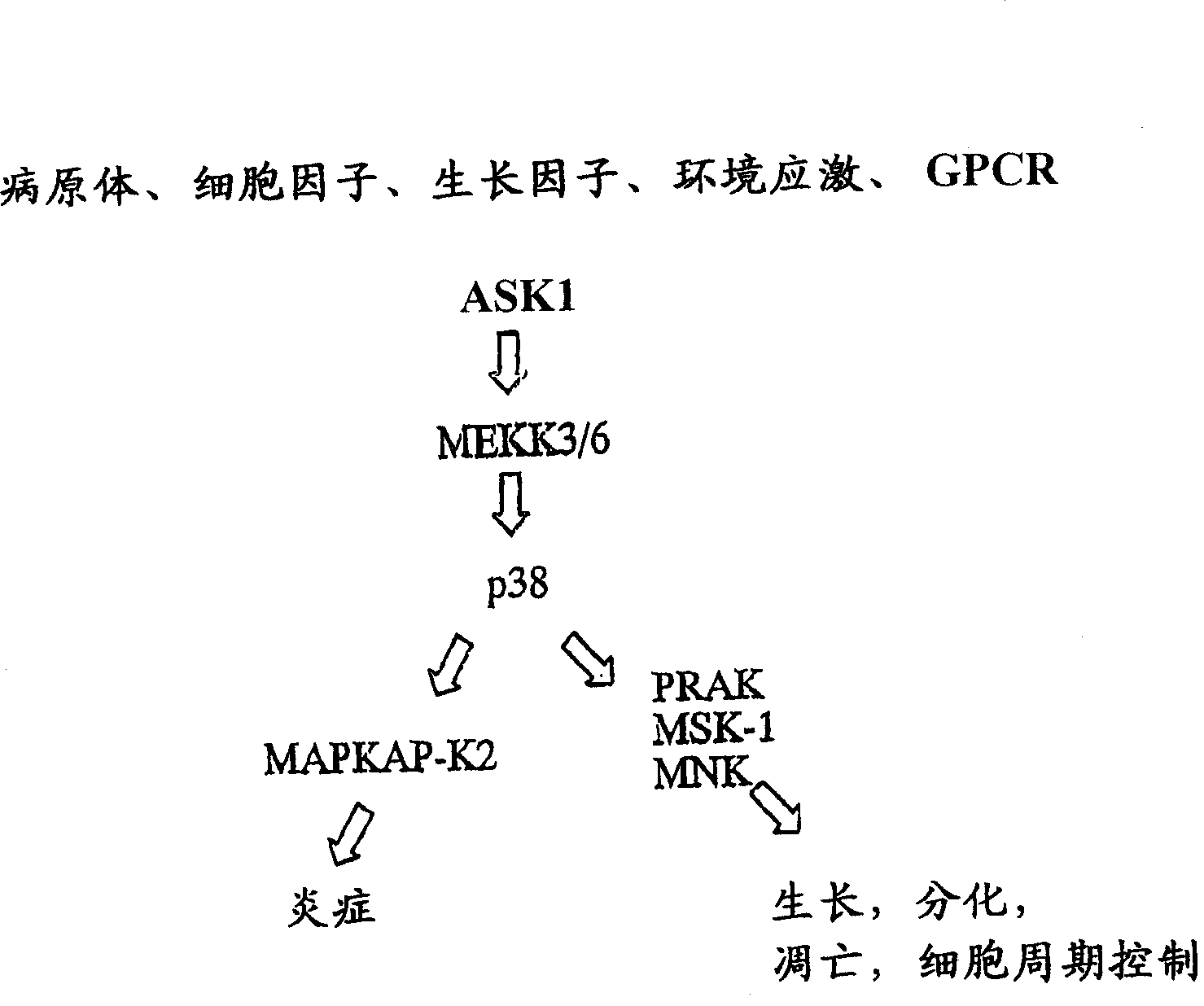
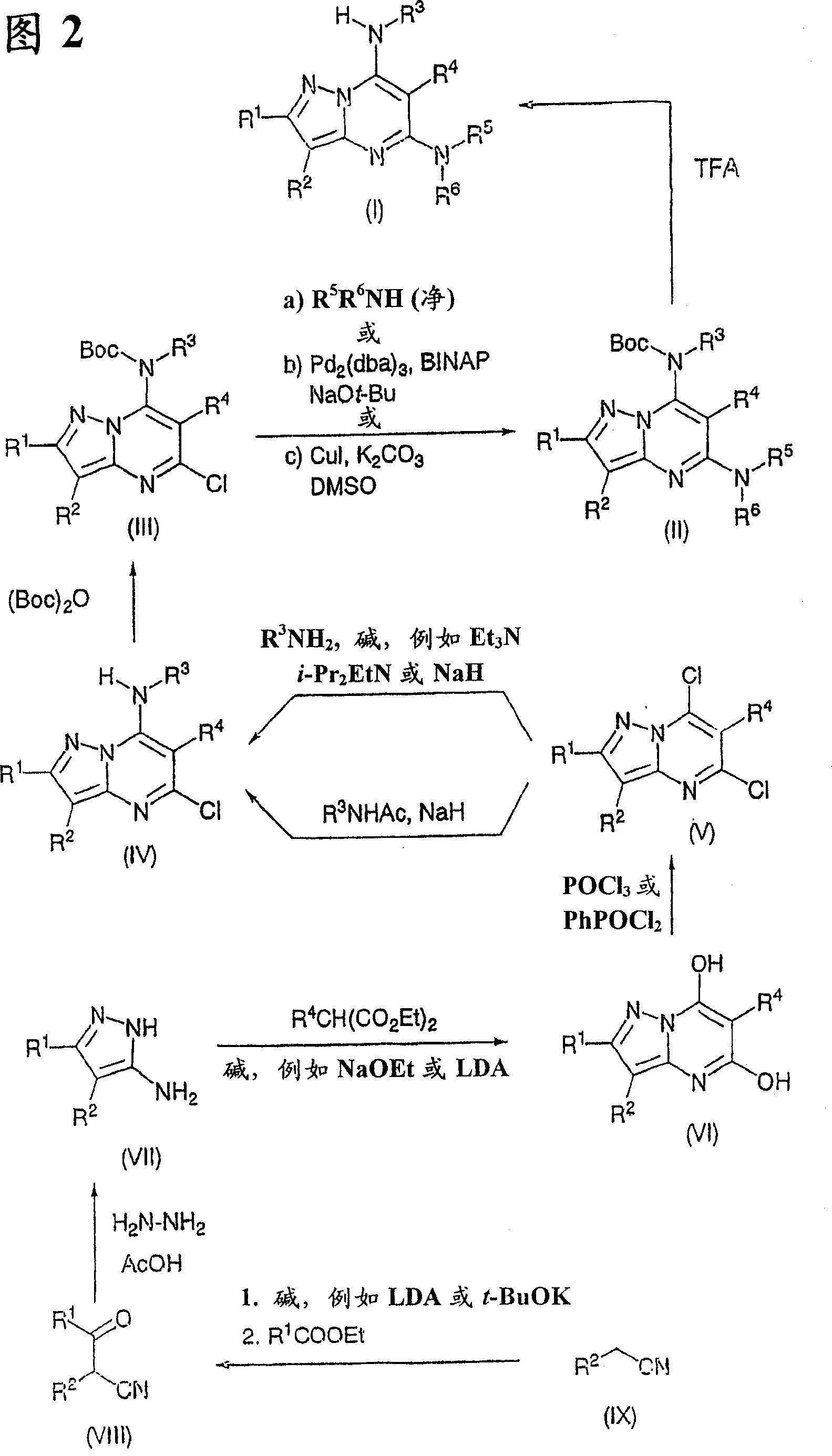
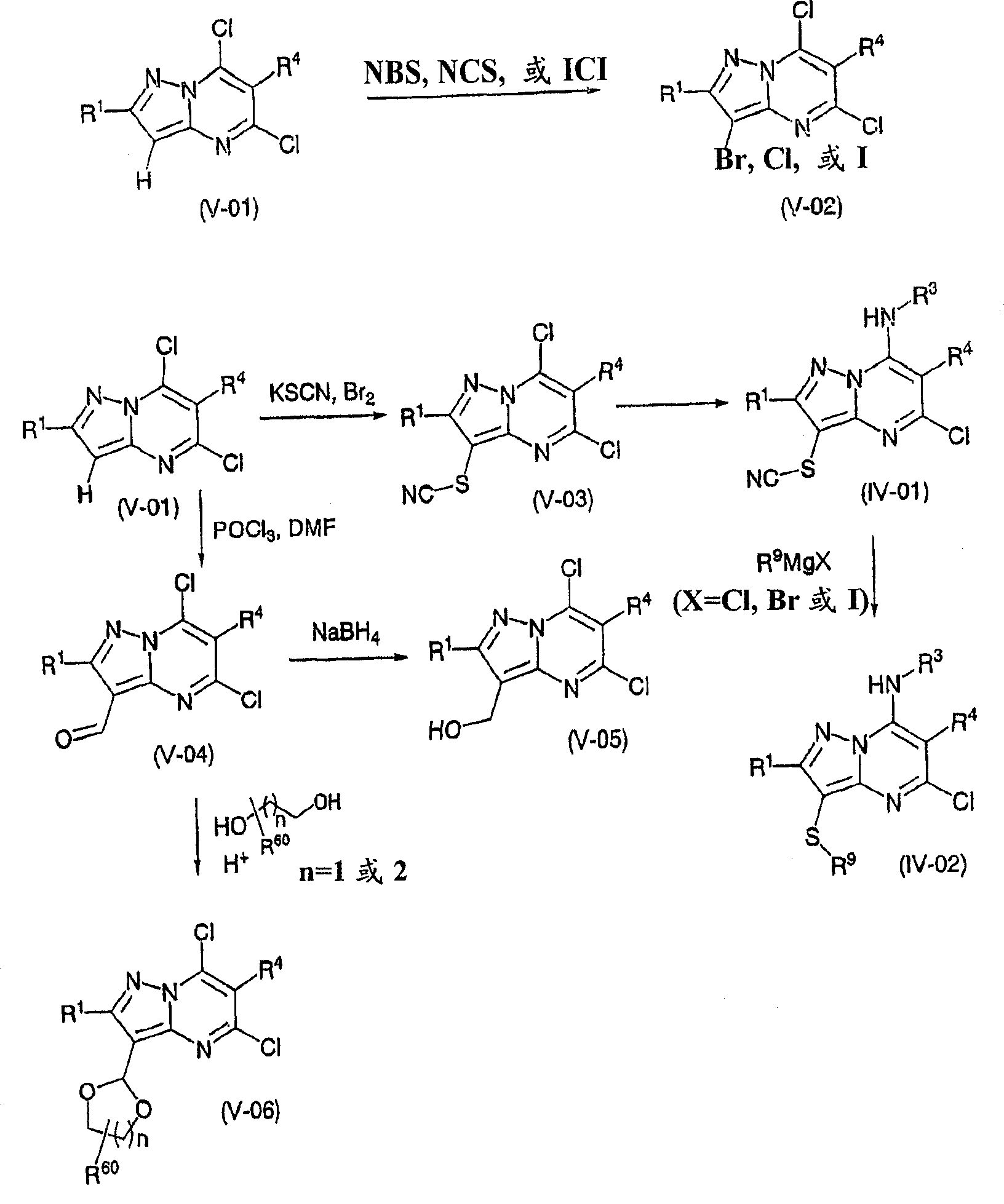
Pyrazolo 1,5-a pyrimidine derivatives
A derivative and biological technology, applied in the field of preparing the compound, preparing the composition, preventing and/or treating various diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0496] [General method for synthesizing pyrazolo[1,5-a]pyrimidines of general formula (VI)]
[0497] To a stirred solution of sodium ethoxide (50mmol) in ethanol (100ml) was added the appropriate 2-substituted malonate diester (20mmol) and the appropriately substituted 3-aminopyrazole (VII) (20mmol). The mixture was heated at reflux for 18 hours, during which time a precipitate formed * . The reaction was cooled to room temperature, then the mixture was filtered through an A4 sinter filter (while washing with a small amount of cold ethanol). The residue was dried in vacuo. The dried precipitate was dissolved in water (about 100 ml), and the resulting solution was acidified (pH 2) with concentrated HCl. This gives a milky white precipitate (VI), which is filtered and dried. Typically non-optimized yields range from 20-40%.
[0498] * In several cases, no or very little precipitation occurred when the substituent was an alkyl chain. In these cases, ethanol was removed und...
Embodiment 2
[0502] [General method for synthesizing pyrazolo[1,5-a]pyrimidines of general formula (V)]
[0503] To a suspension of dihydroxy compound (VI) (2 g) in N,N-dimethylaniline (2 ml) was added phosphoryl chloride (or phenylphosphonyl dichloride) (20 ml). The mixture was heated at reflux for 18 hours, then excess phosphoryl chloride (or phenylphosphonyl dichloride) was removed in vacuo. The residue was poured into ice (50 g) and washed with CH 2 Cl 2 Extraction (5x). The organic phase was adsorbed on neutral (active I) alumina and purified by chromatography (typically using petroleum ether→30% ethyl acetate / petroleum ether as eluent). Appropriately substituted 5,7-dichloropyrazolo[1,5-a]pyrimidine intermediate (V) was obtained in about 40% yield.
[0504]
[0505] Compound number R 1 R 2 R 4 mp(°C) or
Embodiment 3
[0507] [General method for synthesizing pyrazolo[1,5-a]pyrimidines of general formula (V-02)]
[0508] At room temperature, a solution of 5,7-dichloropyrazolo[1,5-a]pyrimidine (V-01) (0.01mol) in chloroform (50ml) was treated with N-chlorosuccinimide, N-bromo Succinimide or iodine monochloride (0.011mol) treatment. The mixture was boiled at reflux until all solids had dissolved and no starting material remained (by TLC). The mixture was poured into ice / water, then the organic layer was separated and washed with Na 2 CO 3 Washed with aqueous solution, over MgSO 4 Dry and remove solvent in vacuo. The residue was purified by silica gel chromatography to give 3-halo-5,7-dichloropyrazolo[1,5-a]pyrimidine (V-02).
[0509] Compound number R 1 R 2 R 4 1 H-NMR (400MHz, CDCl 3 )d(ppm) V-11 H Br H 8.2(s, 1H, Het-H), 7.05(s, 1H, Het-H). V-12 H I H 8.15(s, 1H, Het-H), 2.60(s, 3H, 6-Me).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com