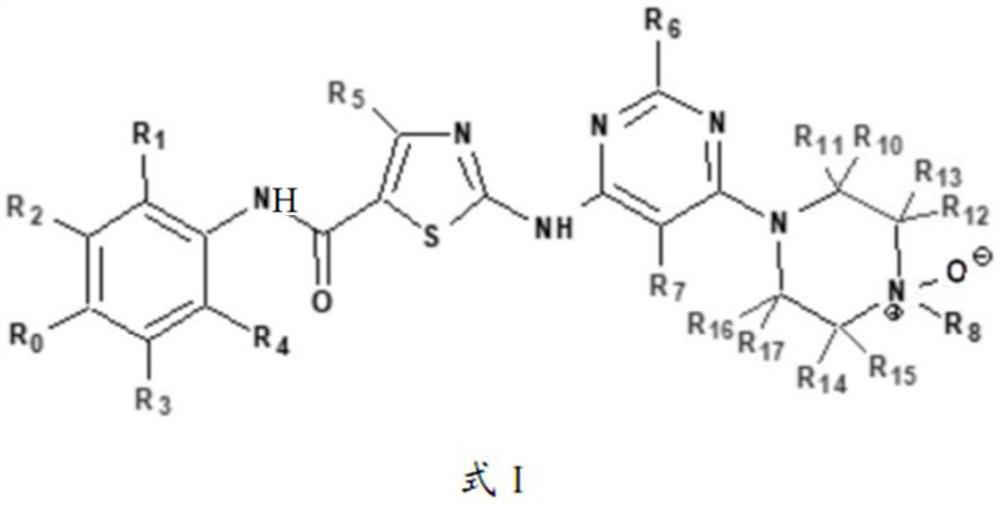
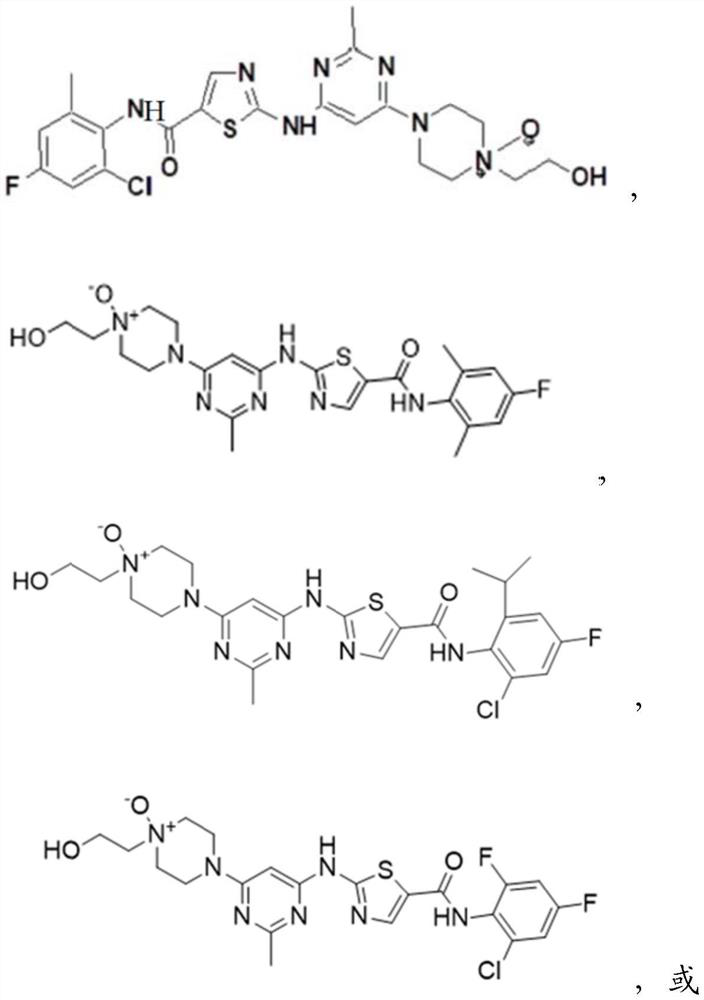
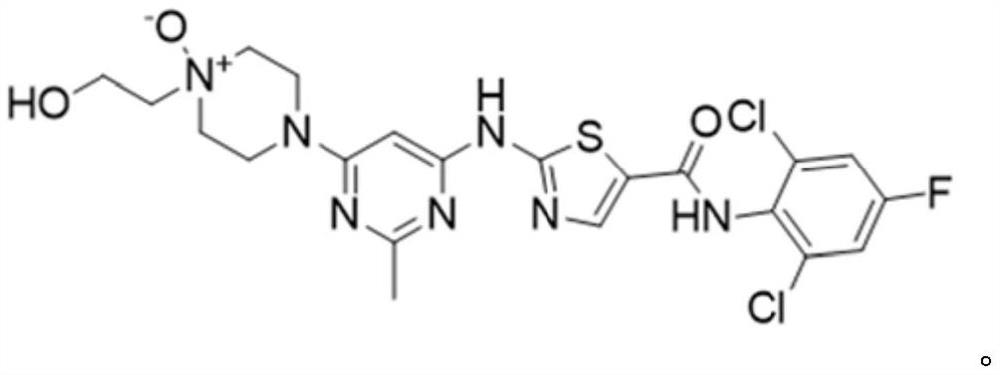
Kinase inhibitor as well as preparation, pharmaceutical composition and application thereof
A compound and solvate technology, applied in the field of medicinal chemistry, can solve problems such as loss of activity, mutation of target protein, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0144] Synthesis of Compound 1:
[0145]
[0146] The synthetic route of compound 1 is as follows:
[0147] Synthesis of [5-(2-chloro-4-fluoro-6-methylphenylcarbamoyl)thiazol-2-yl]-carbamic acid tert-butyl ester
[0148]
[0149] N 2 Under gas protection, add 24.4g (0.1mol) of 2-tert-butoxycarbonylaminothiazole-5-carboxylic acid and 0.5ml of DMF (N,N-dimethylformamide) into 250ml of dichloromethane, and slowly add 13ml of Oxalyl chloride solution (0.15mol), reacted for 2h, and removed the solvent by rotary evaporation to obtain a white solid, which was dissolved in 100ml of anhydrous dichloromethane, and slowly added dropwise to 2-chloro-4-fluoro -6-methylaniline 17.5g (0.11mol) and N,N-diisopropylethylamine 38.8g (0.3mol) in dichloromethane solution, N 2 React at room temperature for 10 h under gas protection, distill off the solvent under reduced pressure, add a mixed solvent of 25 ml of ethyl acetate and 25 ml of n-hexane and stir for 2 hours, filter with suction, ...
preparation example 2
[0163] Synthesis of Compound 2:
[0164]
[0165] The synthetic route of compound 2 is as follows:
[0166]Synthesis of 2-(4-(6-chloro-2-methylpyrimidin-4-yl)piperazin-1-yl)ethanol
[0167]
[0168] 2-(piperazin-1-yl)ethanol (13..0g, 100mmol) and triethylamine (30.3g, 300mmol) were added to 4,6-dichloro-2-dimethylpyrimidine (16.3g, 100mmol ) in 400 mL of dichloromethane solution, stirred at room temperature for 2 hours (LCMS monitoring the reaction). After the reaction was complete, the reaction solution was washed with saturated brine (50ml×1) and water (50ml×1) respectively, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated in vacuo to obtain 30.8g of a yellow powdery solid with a yield of 82%. 68% purity. MS-ESI(m / Z): [M+H] + , 257.
[0169] Synthesis of 2-((6-(4-(2-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-yl)amino)thiazole-5-carboxylic acid methyl ester
[0170]
[0171] Compound 2-(4-(6-chloro-2-methylpyrimidin-4-yl)pi...
preparation example 3
[0184] Synthesis of compound 3:
[0185]
[0186] The synthetic route of compound 3 is as follows:
[0187] Preparation of 2-((6-(4-(2-acetoxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-yl)amino)thiazole-5 according to compound 2 synthesis steps 1-4 -carboxylic acid.
[0188] Synthesis of Compound 4-Fluoro-2-isopropenylaniline
[0189]
[0190] Under argon protection, 2-bromo-4-fluoroaniline (10.0g, 52.6mmol), isopropenylboronic acid pinacol ester (9.7g, 57.8mmol), K 2 CO 3 (21.7g, 157.8mmol) and Pd 2 (dppf) 2 Cl (3.8g, 5.2mmol) was added to 250ml of 1,4-dioxane / water (10:1) solution, heated to 80°C, and reacted for 25 hours (reaction monitored by TCL). After the reaction was complete, water (100ml) was added and extracted with ethyl acetate (100ml×3). The combined organic phases were dried and concentrated to give the crude product. Ethyl acetate / petroleum ether (50:1) was used to pass through the chromatographic column, and finally 4.5 g of a yellow liquid produc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com