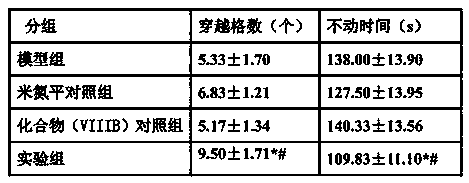
Anti-depressant drug and its oral particulate agent and pharmacy purpose thereof
An anti-depressant and granule technology, applied in the field of medicine, can solve problems such as prolonging the time of taking medicine and increasing the dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Oral granules for the treatment of depression
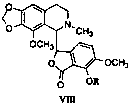
[0036] Prescription of raw and auxiliary materials: mirtazapine 12g, compound (VIIIA) 2g, mannitol 247.2g, hydroxypropyl methylcellulose 96g, aspartame 2.4g and an appropriate amount of purified water.
[0037] Preparation:
[0038] The prescription amount of mirtazapine and compound (VIIIA) were pulverized through 80 mesh sieve to obtain main drug powder; the prescription amount of mannitol, hydroxypropyl methylcellulose and aspartame were respectively pulverized through 60 mesh sieve to obtain excipient powder ; Mix the main ingredient powder and auxiliary material powder evenly, add purified water to prepare soft material, pass through a 30-mesh sieve to granulate, dry in vacuum at 30°C for 60 minutes, granulate with a 24-mesh sieve, and pack separately.
Embodiment 2
[0039] Example 2 Oral granules for the treatment of depression
[0040] Prescription of raw and auxiliary materials: 10 g of mirtazapine, 2 g of compound (VIIIA), 206 g of mannitol, 80 g of hydroxypropyl methylcellulose, 2 g of aspartame and an appropriate amount of purified water.
[0041] Preparation:
[0042] The prescription amount of mirtazapine and compound (VIIIA) were pulverized through 80 mesh sieve to obtain main drug powder; the prescription amount of mannitol, hydroxypropyl methylcellulose and aspartame were respectively pulverized through 60 mesh sieve to obtain excipient powder ; Mix the main ingredient powder and auxiliary material powder evenly, add purified water to prepare soft material, pass through a 30-mesh sieve to granulate, dry in vacuum at 30°C for 60 minutes, granulate with a 24-mesh sieve, and pack separately.
Embodiment 3
[0043] Embodiment 3 oral granules for treating depression
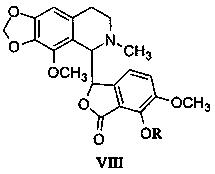
[0044] Prescription of raw and auxiliary materials: 10 g of mirtazapine, 2 g of compound (VIIIB), 206 g of mannitol, 80 g of hydroxypropyl methylcellulose, 2 g of aspartame and an appropriate amount of purified water.
[0045] Preparation method: take the prescribed amount of mirtazapine and compound (VIIIB) and pulverize them through a 80-mesh sieve to obtain the main drug powder; take the prescribed amount of mannitol, hydroxypropyl methylcellulose and aspartame and pulverize them through a 60-mesh sieve respectively, Obtain auxiliary material powder; mix the main drug powder and auxiliary material powder evenly, add purified water to prepare a soft material, granulate through a 30-mesh sieve, vacuum-dry at 30°C for 60 minutes, granulate with a 24-mesh sieve, and pack separately.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com