Synthesis technology for ledipasvir intermediate
A synthesis process and intermediate technology, which is applied in the field of synthesis process of ledipasvir intermediates, can solve problems such as unfavorable reactions and reduced synthesis yield of ledipasvir, and achieve the goals of reducing impurity content, facilitating promotion and reducing costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] The synthetic technique of this ledipasvir intermediate comprises the following steps:
[0014] The first step is raw material selection, and methyl glyoxylate is selected as the raw material for the synthesis of ledipasvir intermediate;
[0015] In the second step, the synthesis of ledipasvir intermediate, add 22g of methyl glyoxylate in a four-neck flask with a thermometer, agitator, and reflux condenser, and then add 12g of R(+)-α- Methylbenzylamine, 200mL of acetic acid, then heated to 58 ° C for 1.5h to obtain a Schiff base compound after the reaction, and then add the Schiff base compound in a four-necked flask with a thermometer, a stirrer, and a reflux condenser, Then add 30mL of cyclopentadiene, 220mL of DMF solvent, 18g of cellulase, and 12g of pectinase, then react at 42°C for 1.2h, and then purify to obtain the intermediate of ledipasvir .
Embodiment 2
[0017] The synthetic technique of this ledipasvir intermediate comprises the following steps:
[0018] The first step is raw material selection, and methyl glyoxylate is selected as the raw material for the synthesis of ledipasvir intermediate;
[0019] In the second step, the synthesis of ledipasvir intermediate, add 24g of methyl glyoxylate in a four-necked flask with a thermometer, agitator, and reflux condenser, and then add 13g of R(+)-α- Methylbenzylamine, 210mL of acetic acid, then heated to 58 ° C for 2h to obtain a Schiff base compound after the reaction, then add the Schiff base compound in a four-necked flask with a thermometer, a stirrer, and a reflux condenser, and then Add 30mL of cyclopentadiene, 220mL of DMF solvent, 18g of cellulase, and 12g of pectinase, and then react at 42°C for 1.6h, and then purify to obtain the intermediate of ledipasvir.
Embodiment 3
[0021] The synthetic technique of this ledipasvir intermediate comprises the following steps:
[0022] The first step is raw material selection, and methyl glyoxylate is selected as the raw material for the synthesis of ledipasvir intermediate;
[0023] In the second step, the synthesis of ledipasvir intermediate, add 28g of methyl glyoxylate in a four-necked flask with a thermometer, agitator, and reflux condenser, and then add 14g of R(+)-α- Methylbenzylamine, 220mL of acetic acid, then heated to 58 ° C for 2.5h to obtain a Schiff base compound after the reaction, then add the Schiff base compound in a four-necked flask with a thermometer, a stirrer, and a reflux condenser, Then add 30mL of cyclopentadiene, 220mL of DMF solvent, 18g of cellulase, 12g of pectinase, and then react at 42°C for 1.8h, and then purify to obtain the intermediate of ledipasvir .
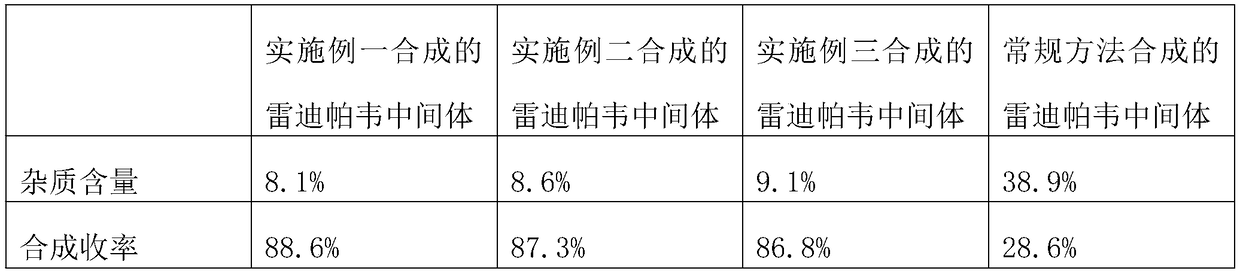
[0024] The intermediates of ledipasvir synthesized in Example 1, Example 2, and Example 3 of the present invention wer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com