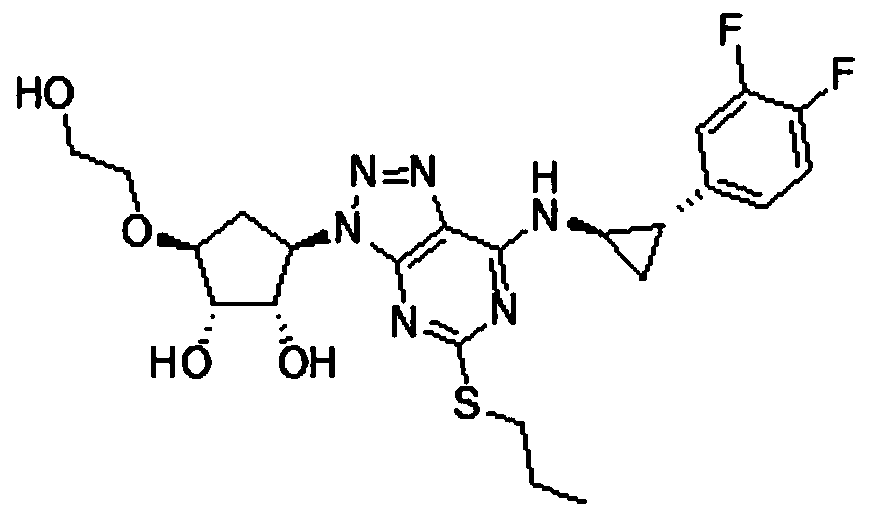
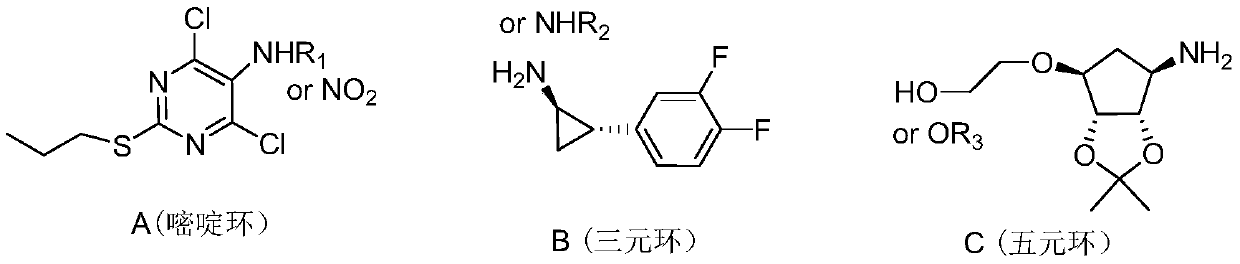
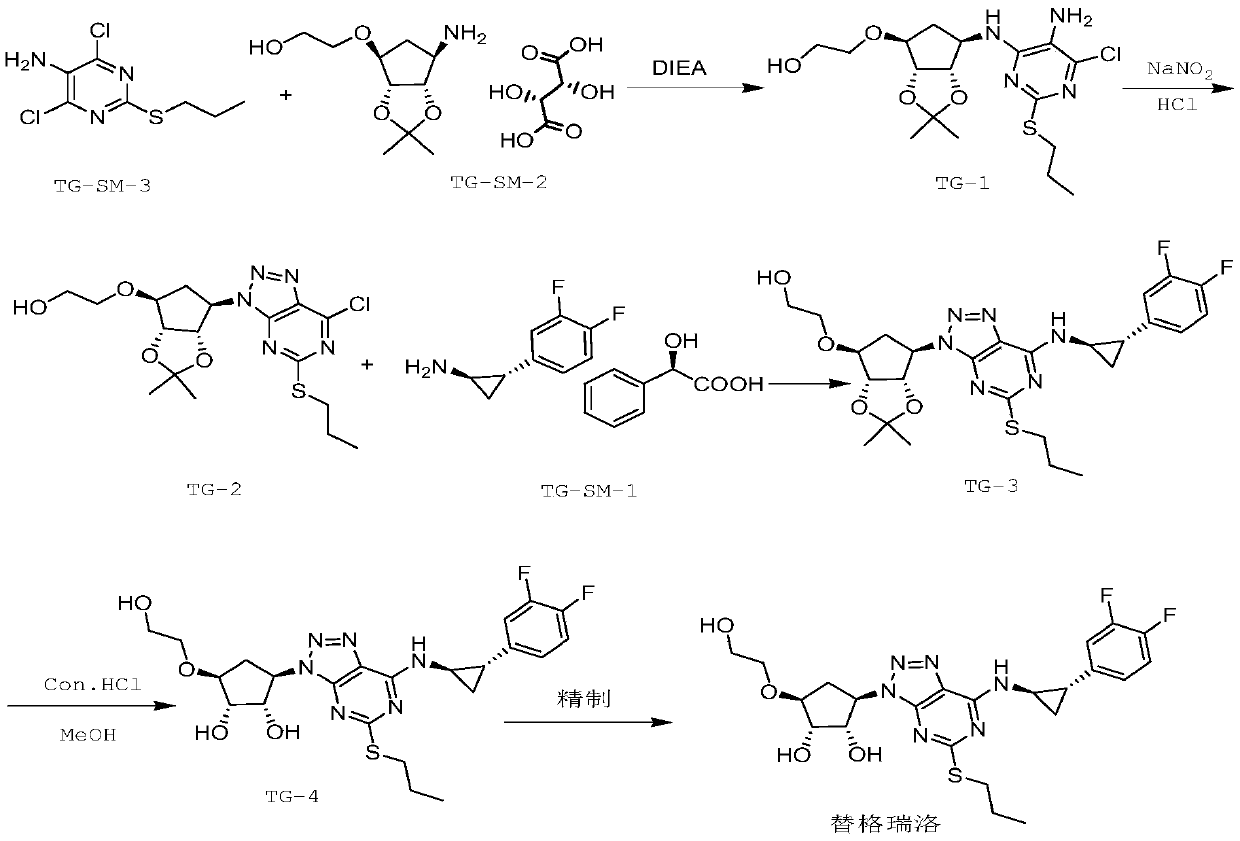
A kind of preparation method of high-purity ticagrelor
A high-purity technology of ticagrelor, applied in the field of drug synthesis, can solve the problems of high consumption, unfavorable environmental protection, high polarity of reaction solvent, poor reaction selectivity, etc., and achieve the effects of easier quality control, shortened reaction time, and increased activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] A preparation method of high-purity ticagrelor, comprising the following steps:
[0055] S1: Preparation of intermediate TG-1:
[0056] Add 8 mol of 1,4-dioxane, 1 mol of TG-SM-3, 1 mol of TG-SM-2 to the reactor in sequence, then add 1 mol of catalyst and 2 mol of N,N-diisopropylethylamine in sequence, and then blow nitrogen into it, and raise the temperature to 90°C Reflux, keep stirring for 8 hours, concentrate under reduced pressure to remove 1,4-dioxane, add dichloromethane and water after cooling down to room temperature, separate liquids, wash the organic layer with water and saturated sodium chloride solution in sequence, and wash the organic layer Concentrate under reduced pressure to obtain the crude product, add ethyl acetate and stir until completely dissolved, heat up and reflux, add n-hexane 4 times the mass of the crude product dropwise to the solution, cool to 15°C to crystallize for 2 hours, filter, and dry in vacuo to obtain off-white solid TG -1;
[...
Embodiment 2
[0074] A preparation method of high-purity ticagrelor, comprising the following steps:
[0075] S1: Preparation of intermediate TG-1:
[0076] Add 25 mol 1,4-dioxane, 1.5 mol TG-SM-3, 1 mol TG-SM-2 to the reactor in sequence, then add 3 mol catalyst and 6 mol N,N-diisopropylethylamine in sequence, pass in nitrogen, and raise the temperature by 100 Reflux at ℃, keep stirring for 10 hours, concentrate under reduced pressure to remove 1,4-dioxane, add dichloromethane and water after cooling down to room temperature, separate liquids, wash the organic layer with water and saturated sodium chloride solution in sequence, and the organic layer after washing Concentrate the layer under reduced pressure to obtain the crude product, add ethyl acetate and stir until it is completely dissolved, raise the temperature and reflux, add n-hexane 5 times the mass of the crude product dropwise to the solution, cool to 18°C to crystallize for 2.5h, filter, and dry in vacuo to obtain off-white ...
Embodiment 3
[0094] A preparation method of high-purity ticagrelor, comprising the following steps:
[0095] S1: Preparation of intermediate TG-1:
[0096] Add 50mol 1,4-dioxane, 2mol TG-SM-3, 1mol TG-SM-2 to the reactor in turn, then add 5mol catalyst and 10mol N,N-diisopropylethylamine in turn, blow nitrogen into it, and raise the temperature to 110°C Reflux, keep stirring for 12 hours, concentrate under reduced pressure to remove 1,4-dioxane, add dichloromethane and water after cooling down to room temperature, separate liquids, wash the organic layer with water and saturated sodium chloride solution in sequence, and wash the organic layer Concentrate under reduced pressure to obtain the crude product, add ethyl acetate and stir until it dissolves completely, heat up and reflux, add n-hexane 6 times the mass of the crude product dropwise to the solution, cool to 20°C to crystallize for 3 hours, filter, and dry in vacuo to obtain off-white solid TG- 1;
[0097] Among them, the molar rati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com