Indeno oxadiazine compound synthetic method
A technology of indenooxadiazine and a synthetic method, applied in directions such as organic chemistry, can solve problems such as low product yield and low purity, and achieve the effect of simplifying operation steps and facilitating industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
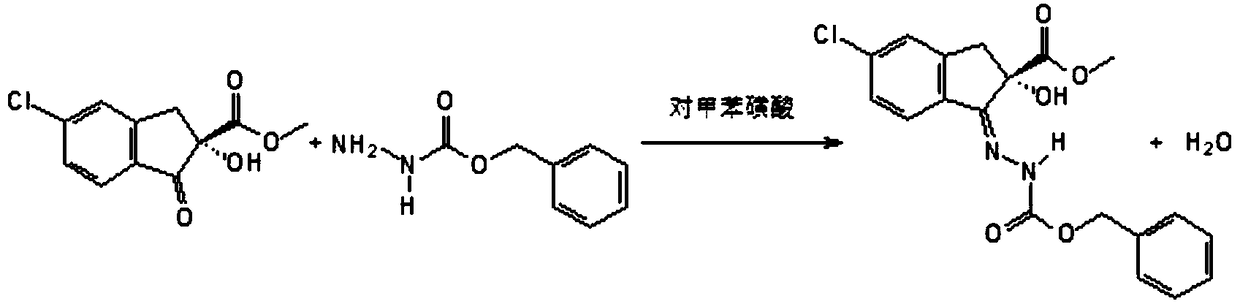
[0023] Synthetic method of 7-chloro-2,3,4a,5-tetrahydro-2-(benzyloxy)carbonylindeno(1,3,4)oxadiazine-4a-carboxylic acid methyl ester of one embodiment , including the following steps:
[0024] S110. Mix toluene, 5-chloro-2,3-dihydro-2-hydroxy-1-oxindene-2-carboxylic acid methyl ester, benzyl carbazate and toluenesulfonic acid at 60°C to 70°C ° C, react under vacuum conditions, remove water while reacting, and obtain the first reaction solution;
[0025] Wherein, the molar ratio of 5-chloro-2,3-dihydro-2-hydroxyl-1-indene-2-carboxylate, benzyl carbazate and toluenesulfonic acid is 1:(1.2~1.5 ): (0.1~0.3).
[0026] Further, the molar ratio of methyl 5-chloro-2,3-dihydro-2-hydroxy-1-oxindene-2-carboxylate, benzyl carbazate and toluenesulfonic acid is 1:1.2:0.1 .
[0027] Further, the vacuum degree of the reaction under vacuum condition is 0.085MPa-0.095MPa.
[0028] Further, the reaction time for the reaction under vacuum condition is 4-8 hours.
[0029] Further, the water ...
Embodiment 1
[0048] Add 50 g (96%, 0.20mol), 40g (0.24mol) of benzyl carbazate, 250g toluene and 3.5g (0.02mol) of anhydrous toluenesulfonic acid, heat up, vacuumize at 60°C, reflux and divide water, and keep warm for 8 hours to obtain The first reaction solution is ready for use.
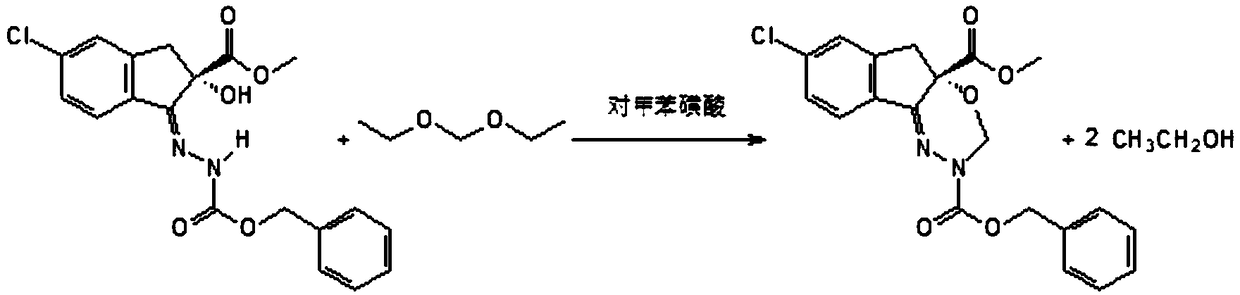
[0049]Add 63 g (0.60 mol) of diethoxymethane and 150 g of toluene to a 1000 mL four-necked flask equipped with a stirrer, a thermometer and a rectifying tower, and gradually add the first reaction solution dropwise to the flask, and pass the rectification while reacting. The column removes the ethanol generated by the reaction, the temperature of the kettle is controlled at 120° C., and the temperature of the top of the rectification column is controlled at 70° C. to obtain the second reaction liquid.
[0050] After removing the toluenesulfonic acid and a small amount of impurities from the second reaction solution through a filter, the toluene and excess diethoxymethane were removed in a vacuum; methanol was ...
Embodiment 2
[0053] Add 50 g (96%, 0.20 mol), 50 g (0.3 mol) of benzyl carbazate, 250 g of toluene and 10.5 g (0.06 mol) of anhydrous toluenesulfonic acid, heat up, vacuumize at 70 ° C, reflux and divide water, and keep warm for 4 hours to obtain The first reaction solution is ready for use.
[0054] Add 105 g (1 mol) of diethoxymethane and 150 g of toluene to a 1000 mL four-necked flask equipped with a stirrer, a thermometer and a rectification tower, gradually add the first reaction solution dropwise to the flask, and pass through the rectification tower while reacting. The ethanol generated by the reaction was removed, the temperature of the kettle was controlled at 100° C., and the temperature of the top of the rectification column was controlled at 80° C. to obtain the second reaction liquid.
[0055] After removing the toluenesulfonic acid and a small amount of impurities from the second reaction solution through a filter, the toluene and excess diethoxymethane were removed in a vac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com