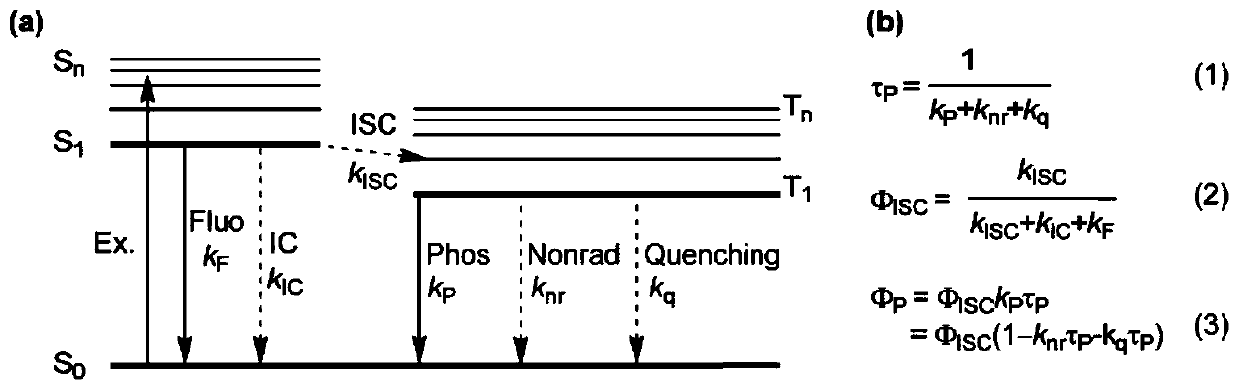
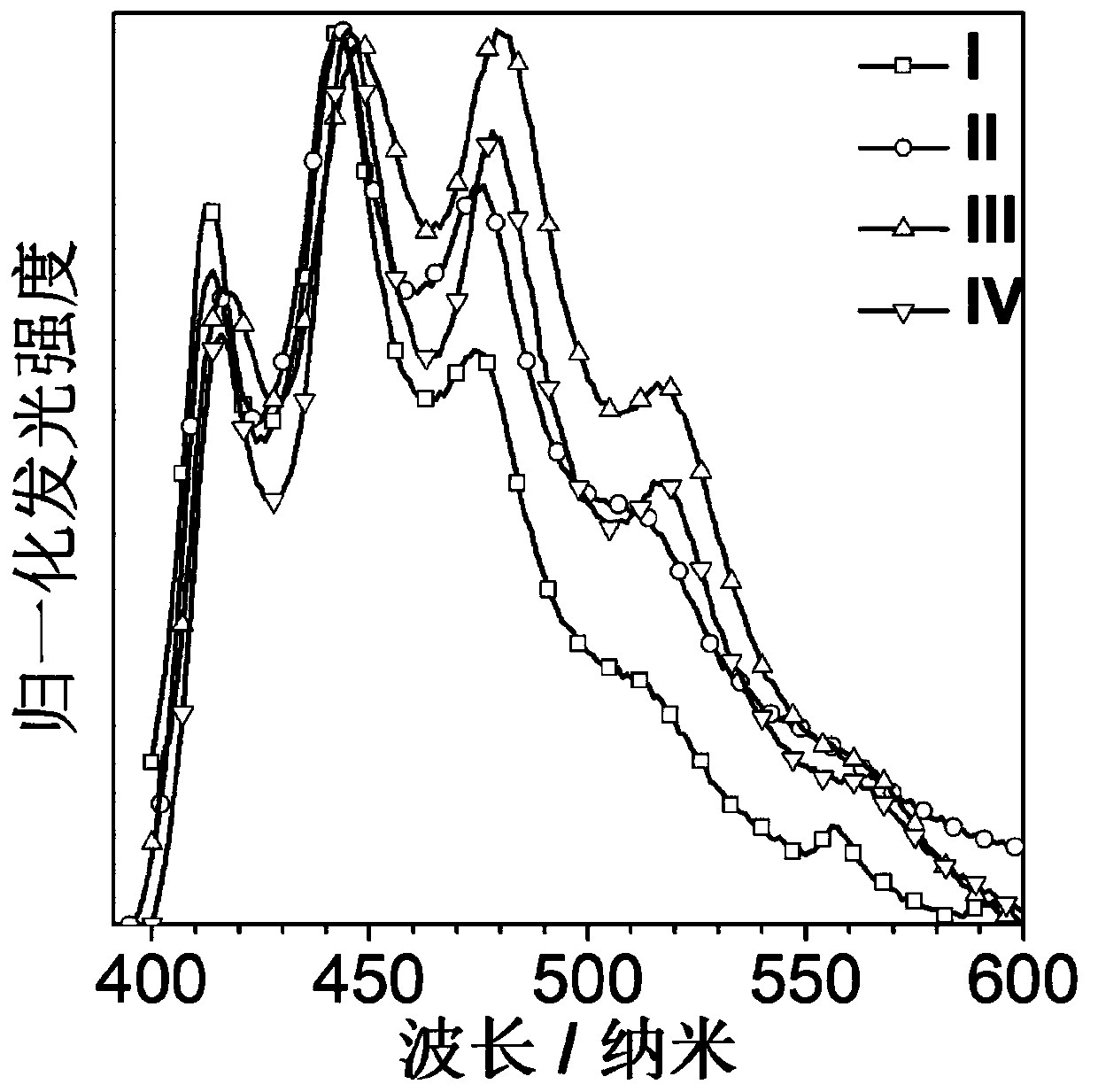
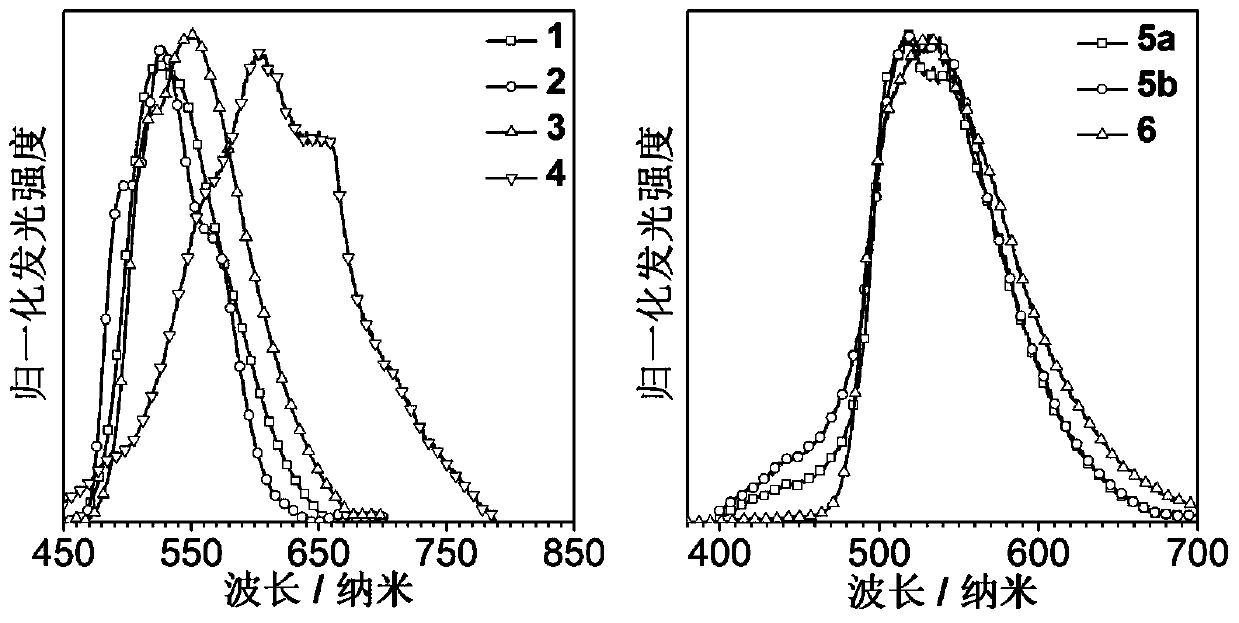
A kind of high-efficiency and long-life organic room temperature phosphorescent material and preparation method thereof
A phosphorescence and compound technology, which is applied in the field of high-efficiency and long-life organic room-temperature phosphorescent materials and their preparation, can solve the problems of weak intermolecular interaction strength, and achieve the effects of low cost, high yield, and great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] When W is an ethoxy group, 4-hydroxy xanthone (5.3g, 25mmol), K 2 CO 3 (6.9g, 50mmol), ethyl bromide (2.23mL, 30mmol) were added to a 250mL two-necked flask, an appropriate amount of acetone solvent was added, and refluxed under nitrogen atmosphere for 12 hours. The reaction was completely cooled to room temperature, and an appropriate amount of dilute hydrochloric acid solution (2mol / L) was added until no bubbles were generated, extracted with chloroform, combined and concentrated organic phases to obtain a crude product, which was purified by chromatography to obtain a pure product. and use 1 H-NMR and 13 The structure was characterized by C-NMR, and it was confirmed that the product was the compound of Example 1. . 1 H-NMR (400MHz, CDCl 3 )δ8.33(dd, J=7.9,1.6Hz,1H),8.25(d,J=8.9Hz,1H),7.73-7.63(m,1H),7.45(d,J=8.3Hz,1H), 7.37(t, J=7.5Hz, 1H), 6.94(dd, J=8.9, 2.3Hz, 1H), 6.87(d, J=2.3Hz, 1H), 4.16(q, J=7.0Hz, 2H), 1.49(t,J=7.0Hz,3H). 13 C NMR (126MHz, CDCl 3 ( ...
Embodiment 2
[0046] When W is a propoxyl group, 4-hydroxy xanthone (5.3g, 25mmol), K 2 CO 3 (6.9g, 50mmol), and bromopropane (2.72mL, 30mmol) were added to a 250mL two-necked flask, an appropriate amount of DMF solvent was added, and refluxed under nitrogen for 5 hours. The reaction was completely cooled to room temperature, and an appropriate amount of dilute hydrochloric acid solution (2mol / L) was added until no bubbles were generated, extracted with chloroform, combined and concentrated organic phases to obtain a crude product, which was purified by chromatography to obtain a pure product. and use 1 H-NMR and 13 The structure was characterized by C-NMR, and it was confirmed that the product was the compound of Example 2. 1 H-NMR (400MHz, CDCl 3 )δ8.22(dd, J=7.9,1.5Hz,2H),8.12(d,J=8.9Hz,2H),7.61-7.49(m,1H),7.60-7.50(m,1H),7.33-7.19 (m,2H),7.37-7.15(m,2H),6.80(dd,J=8.9,2.3Hz,1H),6.80(dd,J=8.9,2.3Hz,1H),6.68(d,J=2.3 Hz,1H),6.68(d,J=2.3Hz,1H),3.90(t,J=6.6Hz,2H),1.87-1.64(m,2H),1.01(t,...
Embodiment 3
[0048] When W is n-butoxy, 4-hydroxy xanthone (5.3g, 25mmol), K 2 CO 3 (6.9g, 50mmol), n-bromobutane (3.23mL, 30mmol) were added to a 250mL two-necked flask, an appropriate amount of acetone solvent was added, and refluxed under nitrogen atmosphere for 12 hours. The reaction was completely cooled to room temperature, and an appropriate amount of dilute hydrochloric acid solution (2mol / L) was added until no bubbles were generated, extracted with chloroform, combined and concentrated organic phases to obtain a crude product, which was purified by chromatography to obtain a pure product. and use 1 H-NMR and 13 The structure was characterized by C-NMR, and it was confirmed that the product was the compound of Example 3. 1 H NMR (400MHz, CDCl 3 )δ8.32(dd, J=7.9,1.6Hz,1H),8.24(d,J=8.9Hz,1H),7.68(ddd,J=8.6,7.1,1.7Hz,1H),7.45(dd,J =8.4,0.6Hz,1H),7.36(ddd,J=8.0,7.2,1.0Hz,1H),6.93(dd,J=8.9,2.4Hz,1H),6.87(d,J=2.3Hz,1H) ,1.88-1.77(m,2H),1.58-1.47(m,2H),1.01(t,J=7.4Hz,3H). 13 C NMR ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com