Diphenylamine derivative organic room temperature phosphorescent compound and its preparation method and application
A room temperature phosphorescence and compound technology, applied in the field of materials science, can solve the problems of lack of theoretical guidance for molecular design and insufficient understanding of luminescent mechanism, and achieve the effects of high yield, low cost and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] Preparation method of diphenylamine derivative organic room temperature phosphorescent compound
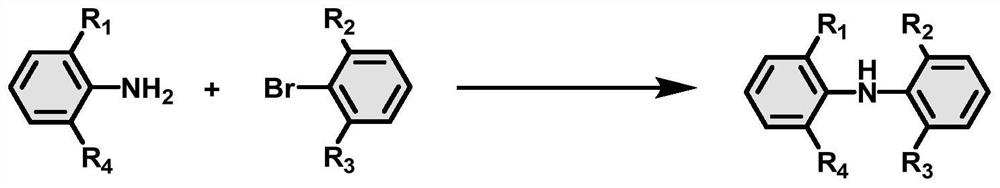
[0036] In the second aspect of the present invention, the present invention provides a method for preparing the diphenylamine derivative organic room temperature phosphorescent compound described in the first aspect of the present invention, and the diphenylamine derivative organic room temperature phosphorescent compound is represented by formula 2 The compound and the compound shown in formula 3 are prepared, and the reaction formula is as follows:
[0037]
[0038] Among them, R 1 , R 2 , R 3 , R 4 Each independently selected from one of hydrogen, C1~C6 alkyl; preferably, R 1 One selected from methyl, isopropyl and tert-butyl; R 2 is hydrogen or methyl; R 3 is hydrogen or methyl; R 4 is hydrogen or methyl.
[0039] In one or more embodiments of the present invention, the preparation method of diphenylamine derivative organic room temperature phosphorescent com...
Embodiment 1
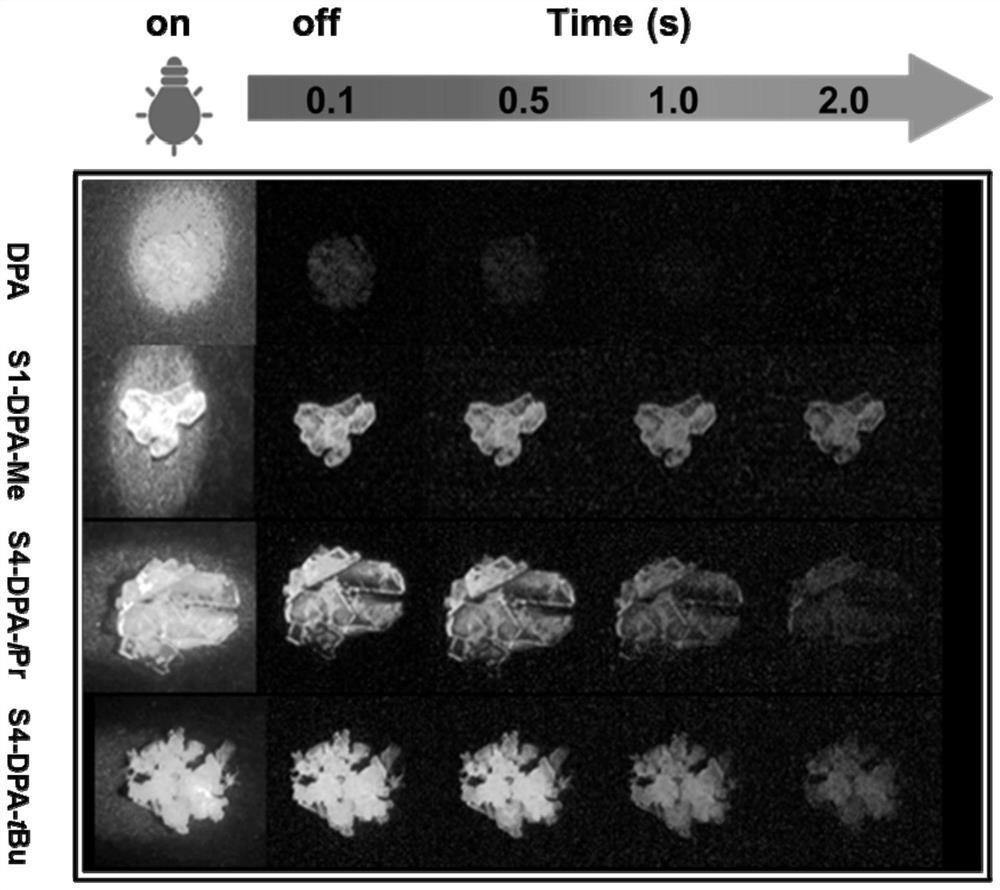
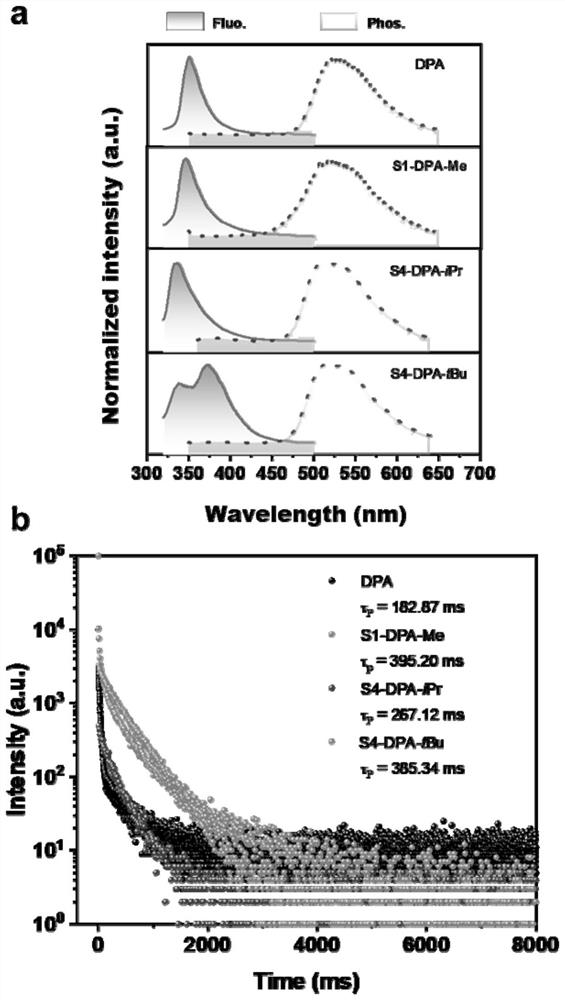
[0052] Example 1: DPA
[0053] The structural formula of compound DPA is as follows:
[0054]
[0055] Compound DPA is commercially available. Using petroleum ether as the eluent, the compound DPA was chromatographed on a silica gel column, separated and purified, and dried in vacuo to obtain a white solid compound DPA, and the H NMR and C NMR spectra ( 1 H NMR, 13 C NMR) characterizes the structure, confirms that this white solid is compound DPA. The test results are as follows:
[0056] DPA: 1 H NMR (400MHz, CDCl 3 )δ7.28(s,1H),7.27(d,J=1.1Hz,2H),7.25(d,J=2.0Hz,1H),7.09(d,J=1.1Hz,3H),7.06(d, J=1.0Hz, 2H), 6.95–6.90(m, 2H), 5.70(s, 1H). 13 C NMR (100MHz, CDCl 3 )δ143.11, 129.34, 120.99, 117.80.
Embodiment 2
[0057] Embodiment 2: Synthesis of S1-DPA-Me
[0058] The structural formula of the target compound S1-DPA-Me is as follows:
[0059]
[0060] The synthesis of S1-DPA-Me comprises the following steps:
[0061] Under nitrogen protection, in a reaction flask, 2-methylaniline (1.62mL, 15mmol, 1eq.), 2-bromobenzene (1.73mL, 16.5mmol, 1.1eq.), NaOtBu (2.88g, 30mmol, 2eq .) and Pd(dppf)Cl 2 ·CH 2 Cl 2 ([1,1'-Bis(diphenylphosphino)ferrocene]palladium dichloride) (0.37 g, 0.45 mmol, 0.03 eq.) was dissolved in sufficient toluene solvent. The mixture was heated to 110° C. with stirring, and the reaction was continued for 16 hours. After the reaction mixture was cooled to room temperature (15-35°C), the reaction was quenched with 1M dilute hydrochloric acid, and then extracted with dichloromethane. The obtained organic layer was washed with saturated aqueous sodium chloride solution (200 mL), dried over anhydrous sodium sulfate Removal of solvent gave crude product. Using petrol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com