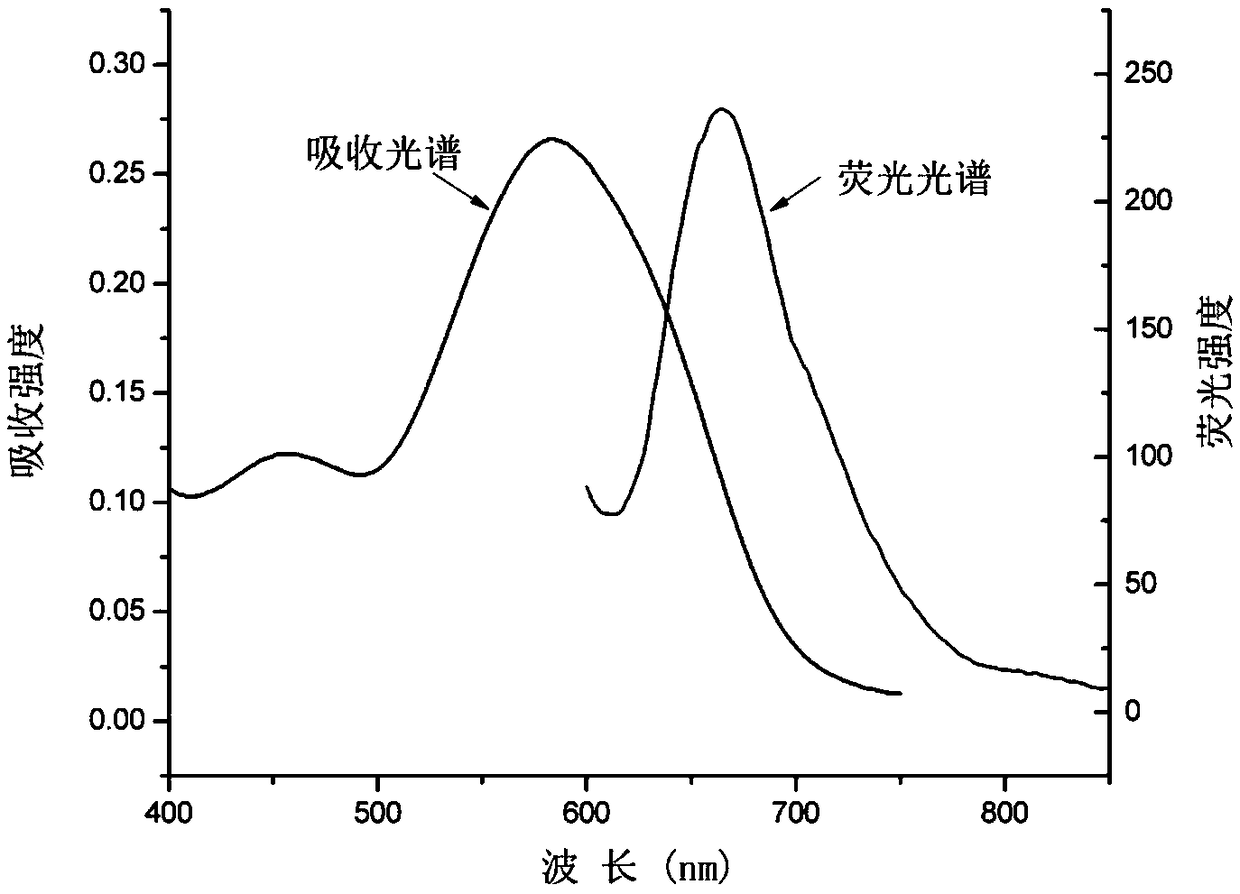
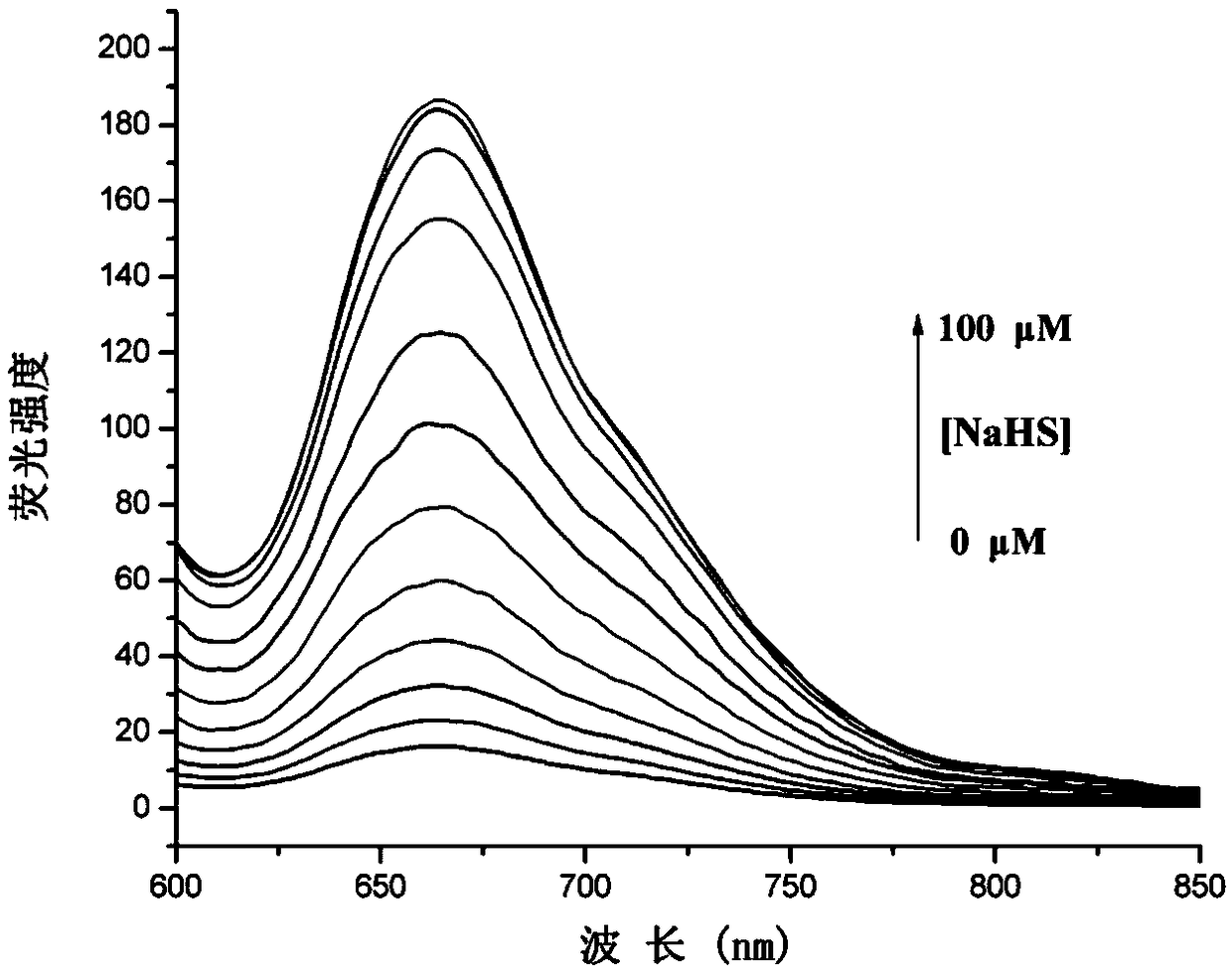
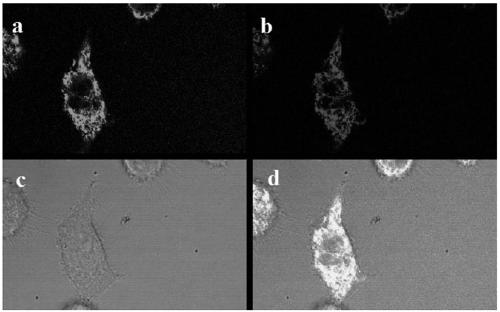
Near-infrared fluorescent dye THX-Np capable of achieving mitochondrial localization and preparation method and application of near-infrared fluorescent dye THX-Np
A fluorescent dye and near-infrared technology, which is applied in the field of dye molecular fluorescent probes, can solve the problems of small anti-interference ability and small Stokes shift, and achieve large Stokes shift, simple preparation method, and good cell membrane communication. The effect of permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of Compound 1
[0049]Add 2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid (5.0g, 16mmol) to 35mL of 98wt% concentrated sulfuric acid containing 3.3mL (31.8mmol) cyclohexanone at 0°C, The reaction was stirred, TLC followed the reaction to the end point, about 1.5h, the reaction solution was cooled to room temperature and poured into 150mL of ice water, 3.5mL of 70wt% perchloric acid was added dropwise, filtered and washed with 100mL of ice water, and the solid was dried to obtain compound 1.
[0050] The synthetic route is as follows:
[0051]
Embodiment 2
[0053] Preparation of fluorescent dye THX-Np
[0054] Compound 1 (3.76g, 10mmol) prepared in Example 1 and 6-hydroxy-2-naphthaldehyde (1.72g, 10mmol) were dissolved in 50ml of glacial acetic acid, stirred and reacted at 95°C, TLC followed the reaction to the end point, about 1h , glacial acetic acid was removed by rotary distillation, and the solid was separated and purified by dichloromethane / methanol (v / v 20:1) column chromatography to obtain the target product as a blue solid (1.34 g, yield: 25%). 1 H NMR(400MHz,DMSO)δ9.84(s,1H),7.96(d,J=7.6Hz,1H),7.86(s,1H),7.80(dd,J=7.8,6.0Hz,2H),7.69 (dd, J=14.5,7.7Hz,2H),7.50(d,J=9.0Hz,2H),7.34(d,J=7.7Hz,1H),7.16-7.06(m,2H),6.58(s, 1H), 6.44(dd, J=23.8, 8.8Hz, 2H), 3.41-3.35(m, 4H), 2.87(d, J=15.5Hz, 1H), 2.70(s, 1H), 1.91(s, 1H ), 1.59(d, J=12.9Hz, 2H), 1.23(s, 1H), 1.10(t, J=6.9Hz, 6H). 13 C NMR(125MHz,DMSO)δ169.44,156.32,135.68,134.14,131.28,130.50,130.40,130.26,129.55,128.92,128.72,128.27,127.99,127.29,126.38,125.24,124.24,119.48...
Embodiment 3
[0058] Preparation of hydrogen sulfide probe
[0059] The near-infrared fluorescent dye (1.06g, 2.0mmol) prepared in Example 1, 2,4-dinitrofluorobenzene (1.12g, 6.0mmol), anhydrous potassium carbonate (0.83g, 6mmol) were dissolved in 60mL dichloro Methane, stirring reaction at room temperature, TLC tracking reaction to the end point, about 2h, the solution color from dark blue to brown, rotary evaporation to remove dichloromethane, solid with dichloromethane / methanol (v / v 20:1) column Chromatographic separation and purification gave the target product as a brown solid (0.53 g, yield: 37%). 1 H NMR (400MHz, DMSO) δ8.95(d, J=2.8Hz, 1H), 8.45(dd, J=9.3, 2.8Hz, 1H), 8.14(d, J=9.0Hz, 1H), 8.08(s ,1H),7.99-7.92(m,2H),7.85-7.76(m,2H),7.69(dd,J=7.9,5.2Hz,2H),7.57(s,1H),7.47(dd,J=8.9 ,2.4Hz,1H),7.35(d,J=7.7Hz,1H),7.29(d,J=9.3Hz,1H),6.57(d,J=2.1Hz,1H),6.44(dt,J=19.1 ,5.6Hz,2H),3.38(d,J=6.7Hz,4H),2.88(d,J=14.2Hz,1H),2.73(d,J=15.6Hz,1H),1.91(d,J=11.9 Hz,1H),1.61(t,J=14.3Hz,3H),1.11(t,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com