Mutant smoothened and methods of using the same
A technology of antibodies and amino acids, applied in the field of smooth mutants and its use, can solve the problems of drug resistance in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0343] 5. Preparation of antibody-drug conjugates
[0344] In an antibody drug conjugate (ADC), an antibody (Ab) is conjugated to one or more drug moieties (D) via a linker (L), for example about 1 to about 20 drug moieties per antibody (p=1 to about 20). ADCs of the formula shown below can be prepared in several ways using organic chemistry reactions, conditions, and reagents known to those skilled in the art, including: (1) reacting the nucleophilic group of the antibody with a divalent linker, thereby utilizing a covalent A valent bond to form Ab-L, followed by reaction with the drug moiety D; and (2) reaction of the nucleophilic group of the drug moiety with a divalent linker, using a covalent bond to form D-L, followed by reaction with the nucleophilic group of the antibody. Additional methods for preparing ADCs are described herein.
[0345] Ab-(L-D) p
[0346] A linker may consist of one or more linker components. Exemplary linker components include: 6-maleimidocap...
example
[0485] Now that the present disclosure is generally described, it will be more readily understood by reference to the following examples, which are presented for purposes of illustration of certain aspects and embodiments of the disclosure and are not intended to limit the disclosure.
example 1
[0486] Example 1: Mutation analysis of vismodegib-resistant basal cell carcinoma.
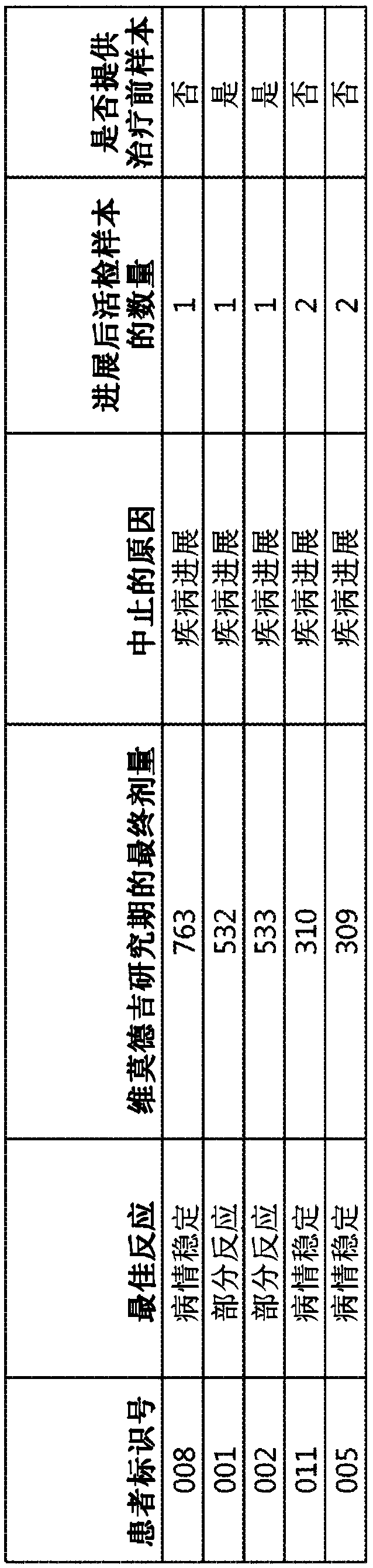
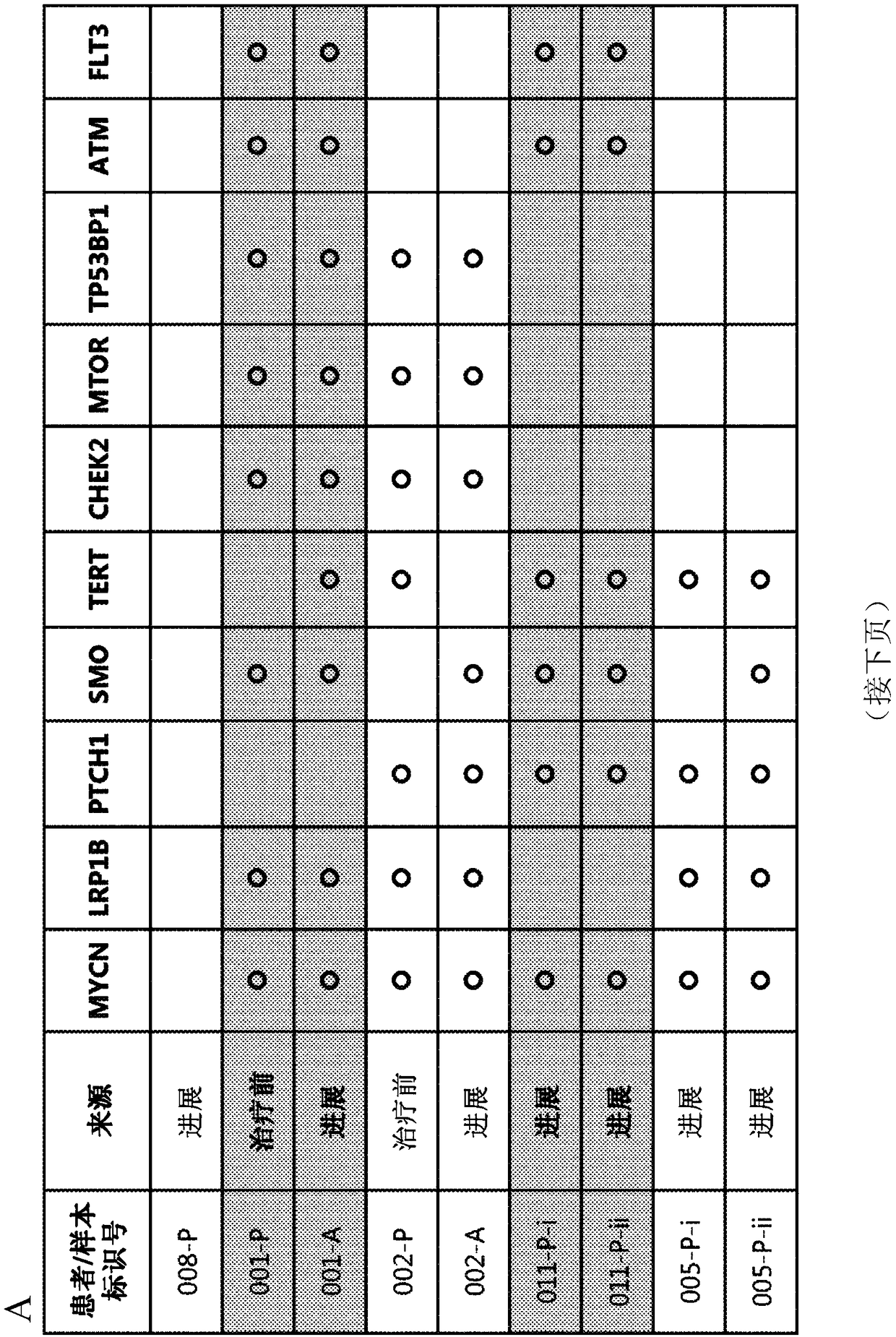
[0487] Clinical responses to targeted therapies (eg, cancer therapy) can be transient due to the acquisition of genetic alterations that confer drug resistance. Identification of resistance mechanisms will guide novel therapeutic strategies. Inappropriate Hh signaling has been linked to several cancers, including basal cell carcinoma (BCC). Loss-of-function mutations in PTCH (about 90%) and activating mutations in SMO (about 10%) are the main pathogenic factors of BCC. By using FoundationOne TM A next-generation sequencing (NGS) platform was used to assess vimodeji sensitivity and the mutation status of patients' BCC to identify clinical mechanisms of resistance to vimodeji (GDC-0449). figure 1 The characteristics of patients with mBCC (metastatic basal cell carcinoma) treated with Vimodeji are listed.
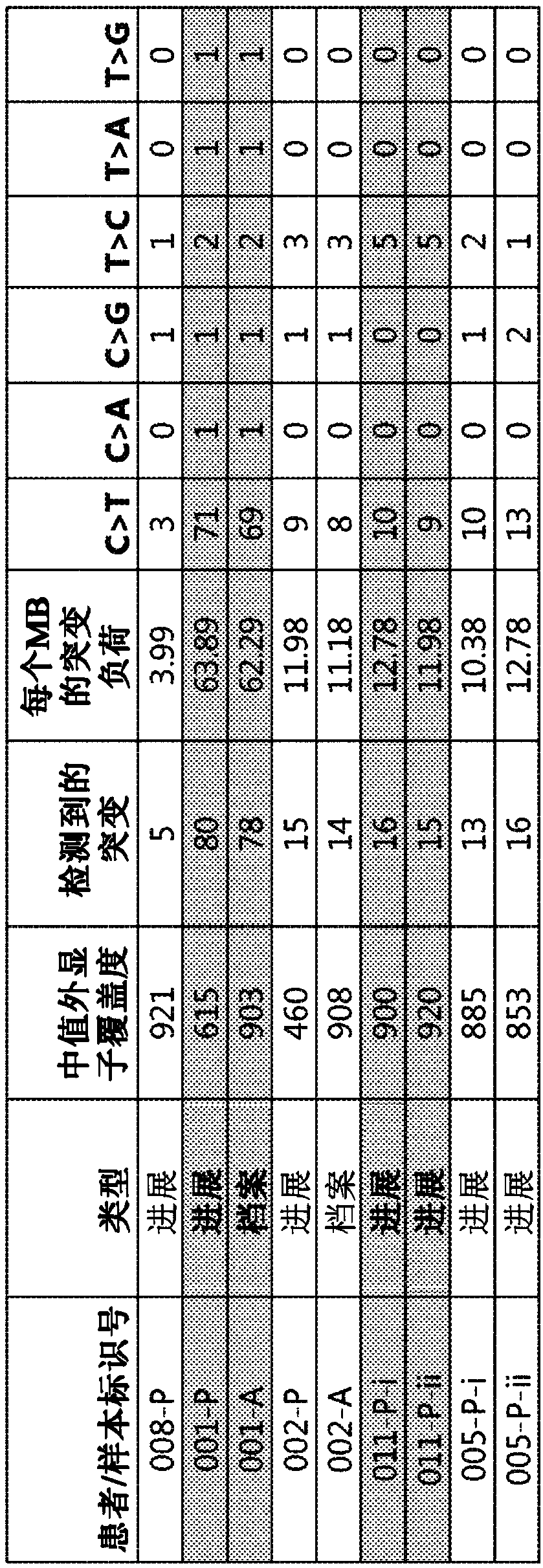
[0488] as in figure 2As shown in , the median exon coverage for each tumor biopsy spe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com