Application and application of reagents for detecting serum exosome pigr in the preparation of kits for diagnosing primary biliary cholangitis and predicting its curative effect
A technology of exosomes and kits, applied in the field of medical detection, can solve the problems of limited means and poor effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
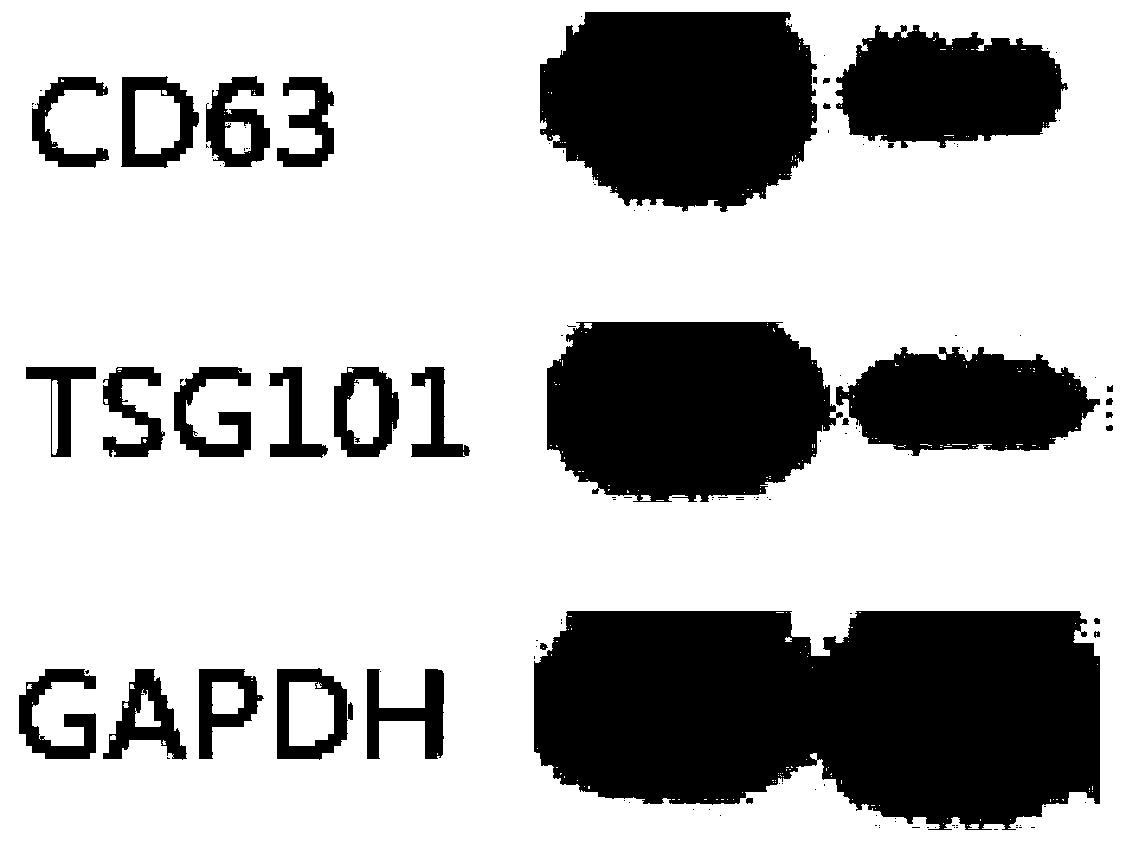
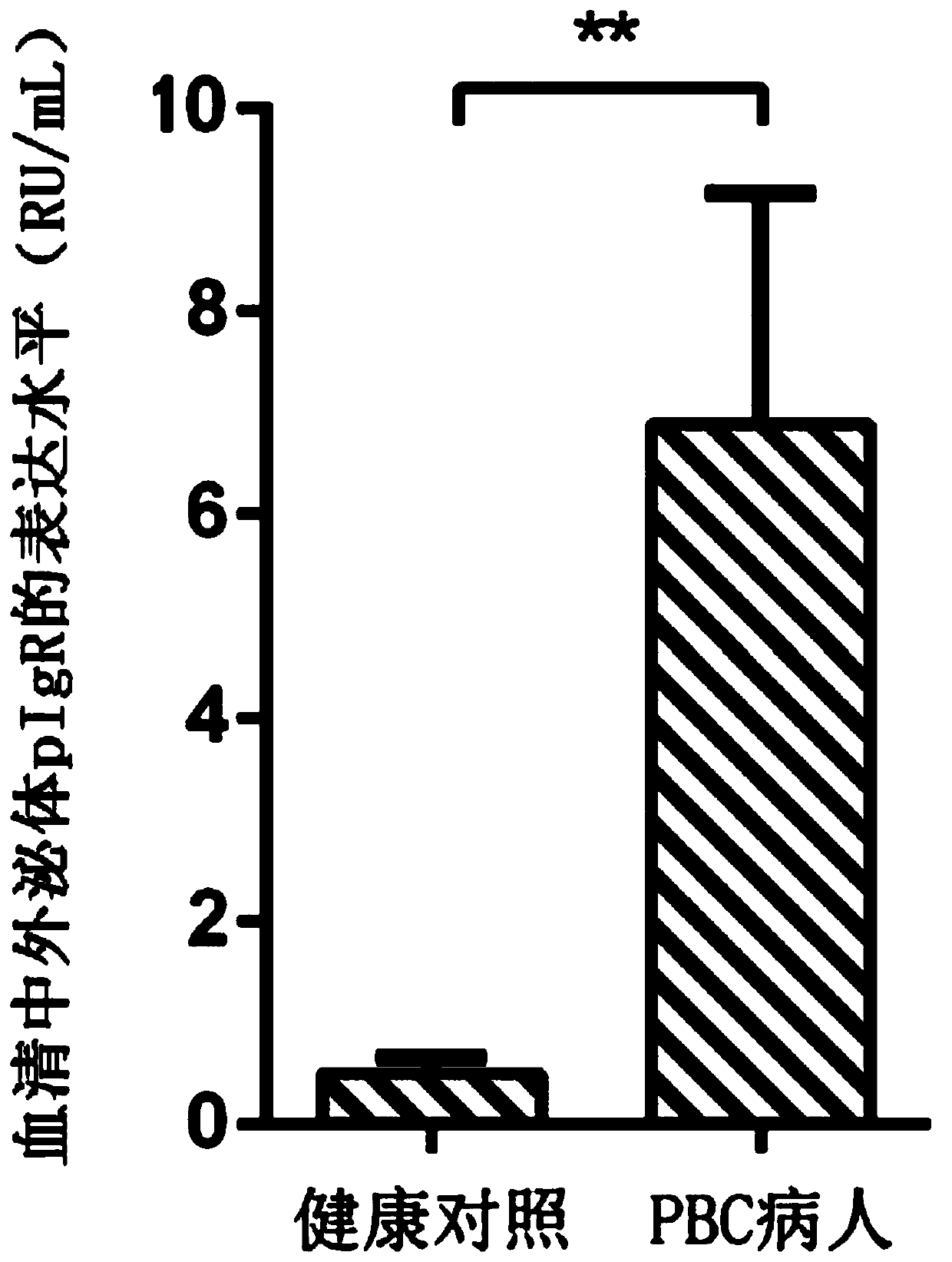
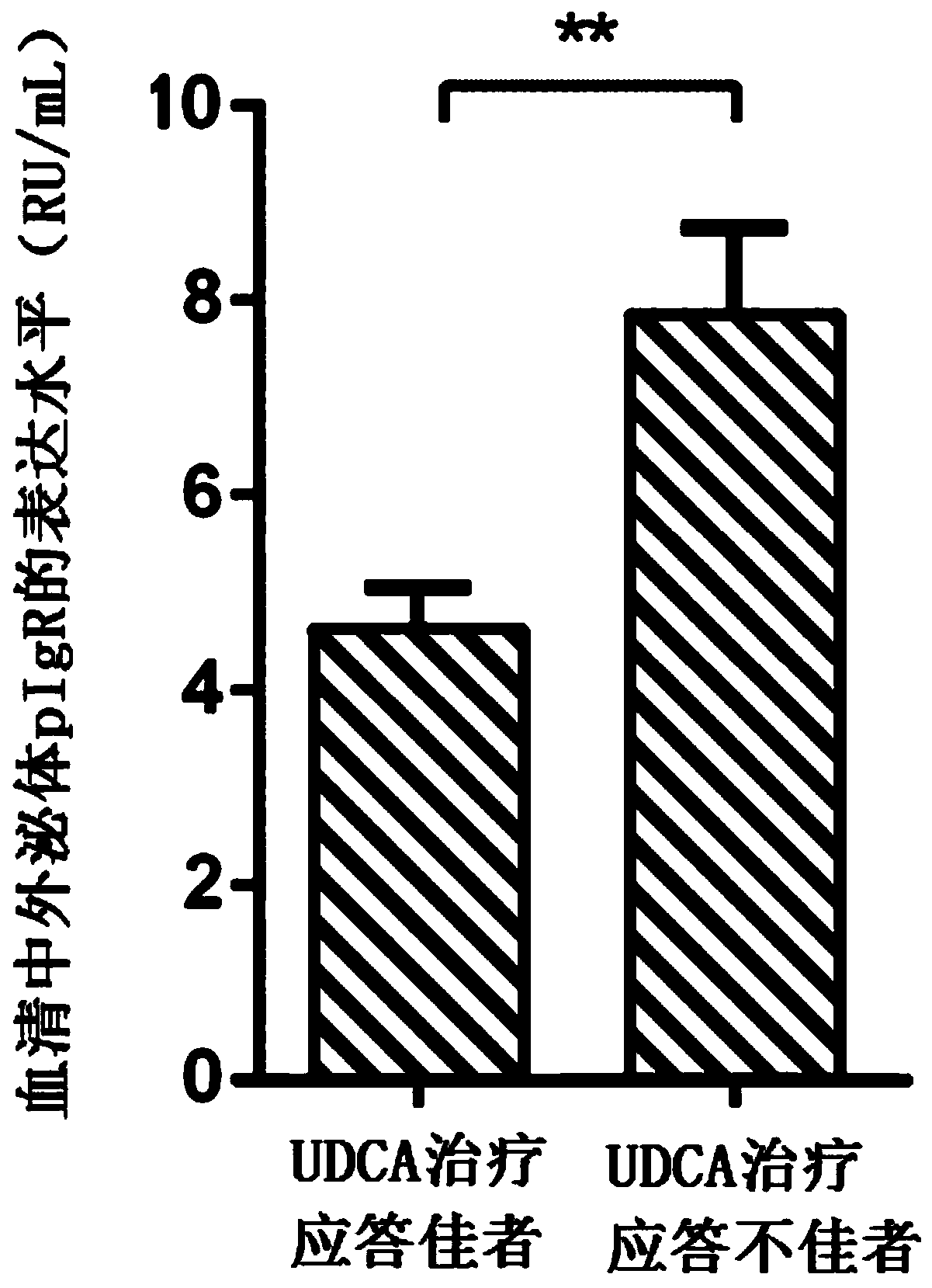
[0032] Application of a reagent for detecting serum exosome pIgR in the preparation of a kit for diagnosing primary biliary cholangitis and predicting its curative effect, the kit includes the following components: pIgR standard; pIgR antigen package coated polystyrene ELISA microplate; enzyme-labeled secondary antibody: horseradish peroxidase (HRP)-labeled anti-human IgG; sample diluent: BSA-PBS solution with a mass concentration of 0.1%; auxiliary reagent: substrate detector color solution, reaction stop solution and wash buffer.
[0033]In this embodiment, the sample diluent is bovine serum albumin added to the 0.01M phosphate buffer solution of pH7.4 and fully dissolved, and 1 gram of bovine serum albumin is added in every liter of phosphate buffer solution; The color solution is TMB / H2O2, the reaction termination solution is: 0.5M sulfuric acid, and the washing buffer solution is Tween20-PBS solution with a mass concentration of 0.05%. The substrate chromogenic solution ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com